Pathologic characteristics of transplanted kidney xenografts
- PMID: 22114174
- PMCID: PMC3269172
- DOI: 10.1681/ASN.2011040429
Pathologic characteristics of transplanted kidney xenografts
Abstract
For xenotransplantation to become a clinical reality, we need to better understand the mechanisms of graft rejection or acceptance. We examined pathologic changes in α1,3-galactosyltransferase gene-knockout pig kidneys transplanted into baboons that were treated with a protocol designed to induce immunotolerance through thymic transplantation (n=4) or were treated with long-term immunosuppressants (n=3). Hyperacute rejection did not occur in α1,3-galactosyltransferase gene-knockout kidney xenografts. By 34 days, acute humoral rejection led to xenograft loss in all three xenografts in the long-term immunosuppression group. The failing grafts exhibited thrombotic microangiopathic glomerulopathy with multiple platelet-fibrin microthrombi, focal interstitial hemorrhage, and acute cellular xenograft rejection. Damaged glomeruli showed IgM, IgG, C4d, and C5b-9 deposition. They also demonstrated endothelial cell death, diffuse endothelial procoagulant activation with high expression of tissue factor and vWF, and low expression of the ectonucleotidase CD39. In contrast, in the immunotolerance group, two of four grafts had normal graft function and no pathologic findings of acute or chronic rejection at 56 and 83 days. One of the remaining kidneys had mild but transient graft dysfunction with reversible, mild microangiopathic glomerulopathy, probably associated with preformed antibodies. The other kidney in the immunotolerance group developed unstable graft function at 81 days and developed chronic xenograft glomerulopathy. In summary, the success of pig-to-primate xenotransplantation may necessitate immune tolerance to inhibit acute humoral and cellular xenograft rejection.
Figures
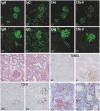





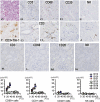
Similar articles
-
Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts.J Am Soc Nephrol. 2005 Sep;16(9):2732-45. doi: 10.1681/ASN.2004121148. Epub 2005 Jul 27. J Am Soc Nephrol. 2005. PMID: 16049072
-
Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons.Am J Pathol. 2008 Jun;172(6):1471-81. doi: 10.2353/ajpath.2008.070672. Epub 2008 May 8. Am J Pathol. 2008. PMID: 18467706 Free PMC article.
-
Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts.Lab Invest. 2000 Jun;80(6):815-30. doi: 10.1038/labinvest.3780086. Lab Invest. 2000. PMID: 10879733
-
Pathology of renal xenograft rejection in pig to non-human primate transplantation.Clin Transplant. 2006;20 Suppl 15:46-52. doi: 10.1111/j.1399-0012.2006.00550.x. Clin Transplant. 2006. PMID: 16848876 Review.
-
Histopathology of xenografts in pig to non-human primate discordant xenotransplantation.Clin Transplant. 2010 Jul;24 Suppl 22:11-5. doi: 10.1111/j.1399-0012.2010.01270.x. Clin Transplant. 2010. PMID: 20590687 Review.
Cited by
-
Xenotransplantation: Current Challenges and Emerging Solutions.Cell Transplant. 2023 Jan-Dec;32:9636897221148771. doi: 10.1177/09636897221148771. Cell Transplant. 2023. PMID: 36644844 Free PMC article. Review.
-
Clinical Pig Kidney Xenotransplantation: How Close Are We?J Am Soc Nephrol. 2020 Jan;31(1):12-21. doi: 10.1681/ASN.2019070651. Epub 2019 Dec 2. J Am Soc Nephrol. 2020. PMID: 31792154 Free PMC article. Review.
-
Advance of genetically modified pigs in xeno-transplantation.Front Cell Dev Biol. 2022 Oct 10;10:1033197. doi: 10.3389/fcell.2022.1033197. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36299485 Free PMC article. Review.
-
Histopathology of pig kidney grafts with/without expression of the carbohydrate Neu5Gc in immunosuppressed baboons.Xenotransplantation. 2021 Nov;28(6):e12715. doi: 10.1111/xen.12715. Epub 2021 Oct 13. Xenotransplantation. 2021. PMID: 34644438 Free PMC article.
-
Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study.Transpl Int. 2018 Oct;31(10):1164-1177. doi: 10.1111/tri.13273. Epub 2018 Jun 5. Transpl Int. 2018. PMID: 29722117 Free PMC article.
References
-
- Sachs DH, Sykes M, Robson SC, Cooper DK: Xenotransplantation. Adv Immunol 79: 129–223, 2001 - PubMed
-
- Cascalho M, Ogle BM, Platt JL: Xenotransplantation and the future of renal replacement. J Am Soc Nephrol 15: 1106–1112, 2004 - PubMed
-
- Cooper DK, Gollackner B, Sachs DH: Will the pig solve the transplantation backlog? Annu Rev Med 53: 133–147, 2002 - PubMed
-
- Sachs DH: The pig as a potential xenograft donor. Vet Immunol Immunopathol 43: 185–191, 1994 - PubMed
-
- Bach FH, Robson SC, Winkler H, Ferran C, Stuhlmeier KM, Wrighton CJ, Hancock WW: Barriers to xenotransplantation. Nat Med 1: 869–873, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

