Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury
- PMID: 22114207
- PMCID: PMC3289418
- DOI: 10.1152/ajprenal.00559.2010
Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury
Abstract
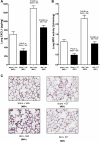
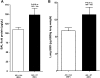
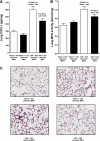
Serum IL-6 is increased in acute kidney injury (AKI) and inhibition of IL-6 reduces AKI-mediated lung inflammation. We hypothesized that circulating monocytes produce IL-6 and that alveolar macrophages mediate lung inflammation after AKI via chemokine (CXCL1) production. To investigate systemic and alveolar macrophages in lung injury after AKI, sham operation or 22 min of renal pedicle clamping (AKI) was performed in three experimental settings: 1) systemic macrophage depletion via diphtheria toxin (DT) injection to CD11b-DTR transgenic mice, 2) DT injection to wild-type mice, and 3) alveolar macrophage depletion via intratracheal (IT) liposome-encapsulated clodronate (LEC) administration to wild-type mice. In mice with AKI and systemic macrophage depletion (CD11b-DTR transgenic administered DT) vs. vehicle-treated AKI, blood monocytes and lung interstitial macrophages were reduced, renal function was similar, serum IL-6 was increased, lung inflammation was improved, lung CXCL1 was reduced, and lung capillary leak was increased. In wild-type mice with AKI administered DT vs. vehicle, serum IL-6 was increased. In mice with AKI and alveolar macrophage depletion (IT-LEC) vs. AKI with normal alveolar macrophage content, blood monocytes and lung interstitial macrophages were similar, alveolar macrophages were reduced, renal function was similar, lung inflammation was improved, lung CXCL1 was reduced, and lung capillary leak was increased. In conclusion, administration of DT in AKI is proinflammatory, limiting the use of the DTR-transgenic model to study systemic effects of AKI. Mice with AKI and either systemic mononuclear phagocyte depletion or alveolar macrophage depletion had reduced lung inflammation and lung CXCL1, but increased lung capillary leak; thus, mononuclear phagocytes mediate lung inflammation, but they protect against lung capillary leak after ischemic AKI. Since macrophage activation and chemokine production are key events in the development of acute lung injury (ALI), these data provide further evidence that AKI may cause ALI.
Figures

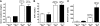
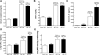



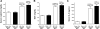

References
-
- Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg 168: 204–214, 2002 - PubMed
-
- Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Dasbach EJ, Platt R. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis 32: 686–693, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

