Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion
- PMID: 22114210
- PMCID: PMC3360581
- DOI: 10.1152/ajprenal.00562.2011
Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion
Abstract
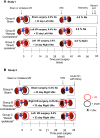
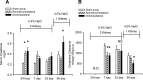
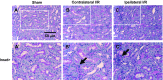
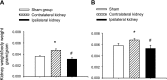
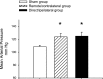
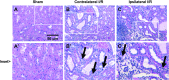
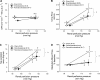
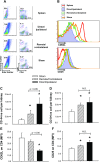
Salt-sensitive hypertension and chronic kidney disease (CKD) following recovery from acute kidney injury (AKI) may occur secondary to incomplete repair, or by activation of circulating factors stimulated by injury. We created two types of renal injury induced by unilateral ischemia-reperfusion (I/R); in a direct/ipsilateral AKI group, rats were subjected to unilateral I/R and the untouched contralateral kidney was removed by unilateral nephrectomy after 5 wk to isolate effects on the injured kidney. In the remote/contralateral AKI group, the injured kidney was removed after 5 wk to isolate effects on the untouched kidney. When these animals were subsequently challenged with elevated dietary sodium for an additional 4 wk (0.4 to 4%), both remote/contralateral and direct/ipsilateral AKI rats manifested a significant increase in blood pressure relative to sham-operated controls. Similarly, in acute studies, both ipsilateral and contralateral kidneys had impaired pressure natriuresis and hemodynamic responses. Reductions in vascular density were observed following direct/ipsilateral injury, but were not observed in the remote/contralateral kidney. However, both remote/contralateral and direct/ipsilateral kidneys contained interstitial cells, some of which were identified as activated (low CD62L/CD4+) T lymphocytes. In contrast, only the direct/ipsilateral AKI group demonstrated significant CKD following exposure to elevated salt. This was characterized by a significant reduction in creatinine clearance, an increase in albuminuria, and a dramatic expansion of interstitial inflammation. Taken together, these data suggest that the salt-sensitive features of AKI on hypertension and CKD are segregable such that effects on hemodynamics and hypertension occur independent of direct renal damage. However, prior direct injury to the kidney is required to elicit the full manifestation of CKD induced by elevated sodium intake.
Figures



References
-
- Anders HJ. Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol 21: 1270–1274, 2010. - PubMed
-
- Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to Ang II following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 289: R1770–R1776, 2005. - PubMed
-
- Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia following ischemia/reperfusion injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003. - PubMed
-
- Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. - PubMed
-
- Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

