Metabolomics in drug target discovery
- PMID: 22114327
- PMCID: PMC4084595
- DOI: 10.1101/sqb.2011.76.010694
Metabolomics in drug target discovery
Abstract
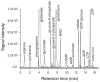
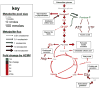
Most diseases result in metabolic changes. In many cases, these changes play a causative role in disease progression. By identifying pathological metabolic changes, metabolomics can point to potential new sites for therapeutic intervention. Particularly promising enzymatic targets are those that carry increased flux in the disease state. Definitive assessment of flux requires the use of isotope tracers. Here we present techniques for finding new drug targets using metabolomics and isotope tracers. The utility of these methods is exemplified in the study of three different viral pathogens. For influenza A and herpes simplex virus, metabolomic analysis of infected versus mock-infected cells revealed dramatic concentration changes around the current antiviral target enzymes. Similar analysis of human-cytomegalovirus-infected cells, however, found the greatest changes in a region of metabolism unrelated to the current antiviral target. Instead, it pointed to the tricarboxylic acid (TCA) cycle and its efflux to feed fatty acid biosynthesis as a potential preferred target. Isotope tracer studies revealed that cytomegalovirus greatly increases flux through the key fatty acid metabolic enzyme acetyl-coenzyme A carboxylase. Inhibition of this enzyme blocks human cytomegalovirus replication. Examples where metabolomics has contributed to identification of anticancer drug targets are also discussed. Eventual proof of the value of metabolomics as a drug target discovery strategy will be successful clinical development of therapeutics hitting these new targets.
Figures
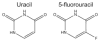

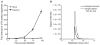


References
-
- Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography–tandem mass spectrometry. J Chromatogr A. 2006;1125:76–88. - PubMed
-
- Beger RD, Hansen DK, Schnackenberg LK, Cross BM, Fatollahi JJ, Lagunero FT, Sarnyai Z, Boros LG. Single valproic acid treatment inhibits glycogen and RNA ribose turnover while disrupting glucose-derived cholesterol synthesis in liver as revealed by the [U-C(6)]-D-glucose tracer in mice. Metabolomics. 2009;5:336–345. - PMC - PubMed
-
- Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–1444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
