A Synthesis of the Tricyclic Core Structure of FR901483 Featuring an Ugi Four-Component Coupling and a Remarkably Selective Elimination Reaction
- PMID: 22114366
- PMCID: PMC3221389
- DOI: 10.1055/s-2008-1042813
A Synthesis of the Tricyclic Core Structure of FR901483 Featuring an Ugi Four-Component Coupling and a Remarkably Selective Elimination Reaction
Abstract
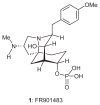
Three key reactions, an efficient Ugi four-component coupling, a regiospecific, base-mediated elimination reaction, and an intramolecular nitrone/alkene [3+2] cycloaddition, were used to achieve an effective synthesis of the tricyclic molecular framework of the immunosuppressant FR901483. The outcome of a control experiment supports the idea that an internal deprotonation by an alkoxide ion is the origin of the site selectivity observed in the base-induced elimination of hydroxy mesylate 17.
Figures
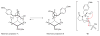





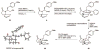
References
-
-
This manuscript is based on Chapter 2 of the Ph.D. thesis of Hirofumi Seike, The Scripps Research Institute, 2003. Experimental procedures and characterization data for the new compounds described in this manuscript are available from the corresponding author upon request.
-
-
- Sakamoto K, Tsujii E, Abe F, Nakanishi T, Yamashita M, Shigematsu N, Izumi S, Okuhara M. J Antibiot. 1996;49:37. - PubMed
-
-
This synthesis established the absolute stereochemistry of FR901483: Snider BB, Lin H. J Am Chem Soc. 1999;121:7778.For an earlier model study, see: Snider BB, Lin H, Foxman BM. J Org Chem. 1998;63:6442.
-
-
- Scheffler G, Seike H, Sorensen EJ. Angew Chem Int Ed. 2000;39:4593. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
