Down-regulation of Shadoo in prion infections traces a pre-clinical event inversely related to PrP(Sc) accumulation
- PMID: 22114562
- PMCID: PMC3219720
- DOI: 10.1371/journal.ppat.1002391
Down-regulation of Shadoo in prion infections traces a pre-clinical event inversely related to PrP(Sc) accumulation
Abstract
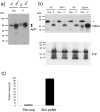
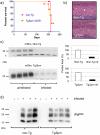
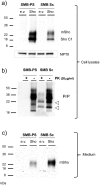
During prion infections of the central nervous system (CNS) the cellular prion protein, PrP(C), is templated to a conformationally distinct form, PrP(Sc). Recent studies have demonstrated that the Sprn gene encodes a GPI-linked glycoprotein Shadoo (Sho), which localizes to a similar membrane environment as PrP(C) and is reduced in the brains of rodents with terminal prion disease. Here, analyses of prion-infected mice revealed that down-regulation of Sho protein was not related to Sprn mRNA abundance at any stage in prion infection. Down-regulation was robust upon propagation of a variety of prion strains in Prnp(a) and Prnp(b) mice, with the exception of the mouse-adapted BSE strain 301 V. In addition, Sho encoded by a TgSprn transgene was down-regulated to the same extent as endogenous Sho. Reduced Sho levels were not seen in a tauopathy, in chemically induced spongiform degeneration or in transgenic mice expressing the extracellular ADan amyloid peptide of familial Danish dementia. Insofar as prion-infected Prnp hemizygous mice exhibited accumulation of PrP(Sc) and down-regulation of Sho hundreds of days prior to onset of neurologic symptoms, Sho depletion can be excluded as an important trigger for clinical disease or as a simple consequence of neuronal damage. These studies instead define a disease-specific effect, and we hypothesize that membrane-associated Sho comprises a bystander substrate for processes degrading PrP(Sc). Thus, while protease-resistant PrP detected by in vitro digestion allows post mortem diagnosis, decreased levels of endogenous Sho may trace an early response to PrP(Sc) accumulation that operates in the CNS in vivo. This cellular response may offer new insights into the homeostatic mechanisms involved in detection and clearance of the misfolded proteins that drive prion disease pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Protease-resistant prions selectively decrease Shadoo protein.PLoS Pathog. 2011 Nov;7(11):e1002382. doi: 10.1371/journal.ppat.1002382. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22163178 Free PMC article.
-
The CNS glycoprotein Shadoo has PrP(C)-like protective properties and displays reduced levels in prion infections.EMBO J. 2007 Sep 5;26(17):4038-50. doi: 10.1038/sj.emboj.7601830. Epub 2007 Aug 16. EMBO J. 2007. PMID: 17703189 Free PMC article.
-
Biological properties of the PrP-like Shadoo protein.Front Biosci (Landmark Ed). 2011 Jan 1;16(4):1505-16. doi: 10.2741/3801. Front Biosci (Landmark Ed). 2011. PMID: 21196244 Review.
-
Function of Prion Protein and the Family Member, Shadoo.Curr Issues Mol Biol. 2020;36:67-88. doi: 10.21775/cimb.036.067. Epub 2019 Sep 27. Curr Issues Mol Biol. 2020. PMID: 31559969
-
Shadoo/PrP (Sprn(0/0) /Prnp(0/0) ) double knockout mice: more than zeroes.Prion. 2012 Nov-Dec;6(5):420-4. doi: 10.4161/pri.21867. Epub 2012 Aug 28. Prion. 2012. PMID: 22929230 Free PMC article. Review.
Cited by
-
Comparative analysis of the Shadoo gene between cattle and buffalo reveals significant differences.PLoS One. 2012;7(10):e46601. doi: 10.1371/journal.pone.0046601. Epub 2012 Oct 10. PLoS One. 2012. PMID: 23071594 Free PMC article.
-
Mice Treated Subcutaneously with Mouse LPS-Converted PrPres or LPS Alone Showed Brain Gene Expression Profiles Characteristic of Prion Disease.Vet Sci. 2021 Sep 21;8(9):200. doi: 10.3390/vetsci8090200. Vet Sci. 2021. PMID: 34564594 Free PMC article.
-
The ZIP-prion connection.Prion. 2012 Sep-Oct;6(4):317-21. doi: 10.4161/pri.20196. Epub 2012 May 17. Prion. 2012. PMID: 22575750 Free PMC article.
-
Unchanged survival rates of Shadoo knockout mice after infection with mouse-adapted scrapie.Prion. 2014;8(5):339-43. doi: 10.4161/19336896.2014.971574. Prion. 2014. PMID: 25495671 Free PMC article.
-
Neuroprotective properties of the PrP-like Shadoo glycoprotein assessed in the middle cerebral artery occlusion model of ischemia.Prion. 2015;9(5):376-93. doi: 10.1080/19336896.2015.1105432. Prion. 2015. PMID: 26516793 Free PMC article.
References
-
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, et al. Transmissions to mice indicate that "new variant" CJD is caused by the BSE agent. Nature. 1997;389:498–501. - PubMed
-
- Manson JC, Cancellotti E, Hart P, Bishop MT, Barron RM. The transmissible spongiform encephalopathies: emerging and declining epidemics. Biochem Soc Trans. 2006;34:1155–1158. - PubMed
-
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, et al. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987;51:651–662. - PubMed
-
- Hunter N, Dann JC, Bennett AD, Somerville RA, McConnell I, et al. Are Sinc and the PrP gene congruent? Evidence from PrP gene analysis in Sinc congenic mice. J Gen Virol. 1992;73:2751–2755. - PubMed
-
- Moore RC, Hope J, McBride PA, McConnell I, Selfridge J, et al. Mice with gene targetted prion protein alterations show that Prnp, Sinc and Prni are congruent. Nat Genet. 1998;18:118–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

