Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation
- PMID: 22114699
- PMCID: PMC3218053
- DOI: 10.1371/journal.pone.0027808
Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation
Abstract
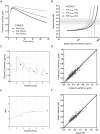
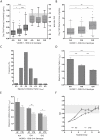
Variable warfarin response during treatment initiation poses a significant challenge to providing optimal anticoagulation therapy. We investigated the determinants of initial warfarin response in a cohort of 167 patients. During the first nine days of treatment with pharmacogenetics-guided dosing, S-warfarin plasma levels and international normalized ratio were obtained to serve as inputs to a pharmacokinetic-pharmacodynamic (PK-PD) model. Individual PK (S-warfarin clearance) and PD (I(max)) parameter values were estimated. Regression analysis demonstrated that CYP2C9 genotype, kidney function, and gender were independent determinants of S-warfarin clearance. The values for I(max) were dependent on VKORC1 and CYP4F2 genotypes, vitamin K status (as measured by plasma concentrations of proteins induced by vitamin K absence, PIVKA-II) and weight. Importantly, indication for warfarin was a major independent determinant of I(max) during initiation, where PD sensitivity was greater in atrial fibrillation than venous thromboembolism. To demonstrate the utility of the global PK-PD model, we compared the predicted initial anticoagulation responses with previously established warfarin dosing algorithms. These insights and modeling approaches have application to personalized warfarin therapy.
Conflict of interest statement
Figures



References
-
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S–198S. - PubMed
-
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–328. - PubMed
-
- Fasco MJ, Principe LM. R- and S-Warfarin inhibition of vitamin K and vitamin K 2,3-epoxide reductase activities in the rat. J Biol Chem. 1982;257:4894–4901. - PubMed
-
- Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

