Genetic and biologic classification of infantile spasms
- PMID: 22114996
- PMCID: PMC3397192
- DOI: 10.1016/j.pediatrneurol.2011.08.010
Genetic and biologic classification of infantile spasms
Abstract
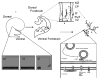
Infantile spasms constitute an age-dependent epilepsy, highly associated with cognitive impairment, autism, and movement disorders. Previous classification systems focused on a distinction between symptomatic and cryptogenic etiologies, and have not kept pace with recent discoveries of mutations in genes in key pathways of central nervous system development in patients with infantile spasms. Children with certain genetic syndromes are much likelier to manifest infantile spasms, and we review the literature to propose a genetic classification of these disorders. Children demonstrating genetic associations with infantile spasms also manifest phenotypes beyond epilepsy that may be explained by recent advances in the understanding of underlying biological mechanisms. Therefore we propose a biologic classification of genes highly associated with infantile spasms, and articulate models for infantile spasms pathogenesis based on those data. The two best described pathways of pathogenesis involve abnormalities in the gene regulatory network of gamma-aminobutyric acidergic forebrain development and abnormalities in molecules expressed at the synapse. These genetic and biologic classifications are flexible, and they should encourage much needed progress in syndrome recognition, clinical genetic testing, and the development of new therapies targeting specific pathways of pathogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–1428. - PubMed
-
- Trevathan E, Murphy CC, Yeargin-Allsopp M. The descriptive epidemiology of infantile spasms among Atlanta children. Epilepsia. 1999;40:748–751. - PubMed
-
- Saemundsen E, Ludvigsson P, Rafnsson V. Risk of autism spectrum disorders after infantile spasms: a population-based study nested in a cohort with seizures in the first year of life. Epilepsia. 2008;49:1865–1870. - PubMed
-
- Philippi H, Wohlrab G, Bettendorf U, Borusiak P, Kluger G, Strobl K, Bast T. Electroencephalographic evolution of hypsarrhythmia: toward an early treatment option. Epilepsia. 2008;49:1859–1864. - PubMed
-
- Watanabe K, Negoro T, Aso K, Matsumoto A. Reappraisal of interictal electroencephalograms in infantile spasms. Epilepsia. 1993;34:679–685. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

