Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases
- PMID: 22115751
- PMCID: PMC3268876
- DOI: 10.1016/j.pharmthera.2011.10.006
Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases
Abstract
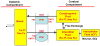
Iron (Fe) and copper (Cu) are essential to neuronal function; excess or deficiency of either is known to underlie the pathoetiology of several commonly known neurodegenerative disorders. This delicate balance of Fe and Cu in the central milieu is maintained by the brain barrier systems, i.e., the blood-brain barrier (BBB) between the blood and brain interstitial fluid and the blood-cerebrospinal fluid barrier (BCB) between the blood and cerebrospinal fluid (CSF). This review provides a concise description on the structural and functional characteristics of the brain barrier systems. Current understanding of Fe and Cu transport across the brain barriers is thoroughly examined, with major focuses on whether the BBB and BCB coordinate the direction of Fe and Cu fluxes between the blood and brain/CSF. In particular, the mechanism by which pertinent metal transporters in the barriers, such as the transferrin receptor (TfR), divalent metal transporter (DMT1), copper transporter (CTR1), ATP7A/B, and ferroportin (FPN), regulate metal movement across the barriers is explored. Finally, the detrimental consequences of dysfunctional metal transport by brain barriers, as a result of endogenous disorders or exogenous insults, are discussed. Understanding the regulation of Fe and Cu homeostasis in the central nervous system aids in the design of new drugs targeted on the regulatory proteins at the brain barriers for the treatment of metal's deficiency or overload-related neurological diseases.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Arredondo M, Munoz P, Mura CV, Nunez MT. DMT-1, a physiologically relevant apical Cu1+ transporter in intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–C1530. - PubMed
-
- Ba LA, Doering M, Burkholz T, Jacob C. Metal trafficking: from maintaining metal homeostasis to future drug design. Metallomics. 2009;1:292–311. - PubMed
-
- Bali PK, Zak O, Aisen P. A new role for the transferrin receptor in the release of iron from transferrin. Biochemistry. 1991;30:324–328. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:204–214. - PubMed
-
- Bertinato J, Iskandar M, L’Abbe MR. Copper deficiency induces the upregulation of copper chaperone for Cu/Zn superoxide dismutase in weanling male rats. Journal of Nutrition. 2003;133:28–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

