Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial
- PMID: 22115874
- PMCID: PMC3242163
- DOI: 10.1016/S0140-6736(11)61125-2
Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial
Abstract
Background: Findings of large randomised trials have shown that lowering LDL cholesterol with statins reduces vascular morbidity and mortality rapidly, but limited evidence exists about the long-term efficacy and safety of statin treatment. The aim of the extended follow-up of the Heart Protection Study (HPS) is to assess long-term efficacy and safety of lowering LDL cholesterol with statins, and here we report cause-specific mortality and major morbidity in the in-trial and post-trial periods.
Methods: 20,536 patients at high risk of vascular and non-vascular outcomes were allocated either 40 mg simvastatin daily or placebo, using minimised randomisation. Mean in-trial follow-up was 5·3 years (SD 1·2), and post-trial follow-up of surviving patients yielded a mean total duration of 11·0 years (SD 0·6). The primary outcome of the long-term follow-up of HPS was first post-randomisation major vascular event, and analysis was by intention to treat. This trial is registered with ISRCTN, number 48489393.
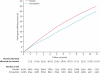
Findings: During the in-trial period, allocation to simvastatin yielded an average reduction in LDL cholesterol of 1·0 mmol/L and a proportional decrease in major vascular events of 23% (95% CI 19-28; p<0·0001), with significant divergence each year after the first. During the post-trial period (when statin use and lipid concentrations were similar in both groups), no further significant reductions were noted in either major vascular events (risk ratio [RR] 0·95 [0·89-1·02]) or vascular mortality (0·98 [0·90-1·07]). During the combined in-trial and post-trial periods, no significant differences were recorded in cancer incidence at all sites (0·98 [0·92-1·05]) or any particular site, or in mortality attributed to cancer (1·01 [0·92-1·11]) or to non-vascular causes (0·96 [0·89-1·03]).
Interpretation: More prolonged LDL-lowering statin treatment produces larger absolute reductions in vascular events. Moreover, even after study treatment stopped in HPS, benefits persisted for at least 5 years without any evidence of emerging hazards. These findings provide further support for the prompt initiation and long-term continuation of statin treatment.
Funding: UK Medical Research Council, British Heart Foundation, Merck & Co, Roche Vitamins.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Statins and safety: can we finally be reassured?Lancet. 2011 Dec 10;378(9808):1980-1981. doi: 10.1016/S0140-6736(11)61544-4. Epub 2011 Nov 22. Lancet. 2011. PMID: 22115875 No abstract available.
-
ACP Journal Club. Simvastatin reduced major vascular events at 5 years in at-risk patients; benefits were maintained at 11 years.Ann Intern Med. 2012 Mar 20;156(6):JC3-5. doi: 10.7326/0003-4819-156-6-201203200-02005. Ann Intern Med. 2012. PMID: 22431691 No abstract available.
-
Safety of long-term simvastatin discontinuation.Lancet. 2012 May 5;379(9827):1704; author reply 1704. doi: 10.1016/S0140-6736(12)60719-3. Lancet. 2012. PMID: 22559898 No abstract available.
-
Mortality and morbidity of lowering low-density lipoprotein cholesterol with simvastatin.Natl Med J India. 2012 Mar-Apr;25(2):90-1. Natl Med J India. 2012. PMID: 22686716 No abstract available.
References
-
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. Eur Heart J. 1999;20:725–741. - PubMed
-
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. - PubMed
-
- Heart Protection Study Collaborative Group The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial. BMC Med. 2005;3:6. - PubMed
-
- Jacobs D, Blackburn H, Higgins M. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86:1046–1060. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

