Interictal spikes, seizures and ictal cell death are not necessary for post-traumatic epileptogenesis in vitro
- PMID: 22115940
- PMCID: PMC3786688
- DOI: 10.1016/j.nbd.2011.11.001
Interictal spikes, seizures and ictal cell death are not necessary for post-traumatic epileptogenesis in vitro
Abstract
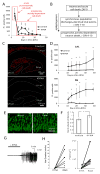
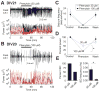
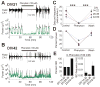
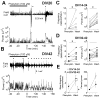
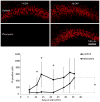
Clinical studies indicate that phenytoin prevents acute post-traumatic seizures but not subsequent post-traumatic epilepsy. We explored this phenomenon using organotypic hippocampal slice cultures as a model of severe traumatic brain injury. Hippocampal slices were cultured for up to eight weeks, during which acute and chronic electrical recordings revealed a characteristic evolution of spontaneous epileptiform discharges, including interictal spikes, seizure activity and electrical status epilepticus. Cell death exhibited an early peak immediately following slicing, and a later secondary peak that coincided with the peak of seizure-like activity. The secondary peak in neuronal death was abolished by either blockade of glutamatergic transmission with kynurenic acid or by elimination of ictal activity and status epilepticus with phenytoin. Withdrawal of kynurenic acid or phenytoin was followed by a sharp increase in spontaneous seizure activity. Phenytoin's anticonvulsant and neuroprotective effects failed after four weeks of continuous administration. These data support the clinical findings that after brain injury, anticonvulsants prevent seizures but not epilepsy or the development of anticonvulsant resistance. We extend the clinical data by showing that secondary neuronal death is correlated with ictal but not interictal activity, and that blocking all three of these sequelae of brain injury does not prevent epileptogenesis in this in vitro model.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

