Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells
- PMID: 22116306
- PMCID: PMC3311237
- DOI: 10.1152/ajpcell.00361.2011
Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells
Abstract
Cyclic AMP signals encode information required to differentially regulate a wide variety of cellular responses; yet it is not well understood how information is encrypted within these signals. An emerging concept is that compartmentalization underlies specificity within the cAMP signaling pathway. This concept is based on a series of observations indicating that cAMP levels are distinct in different regions of the cell. One such observation is that cAMP production at the plasma membrane increases pulmonary microvascular endothelial barrier integrity, whereas cAMP production in the cytosol disrupts barrier integrity. To better understand how cAMP signals might be compartmentalized, we have developed mathematical models in which cellular geometry as well as total adenylyl cyclase and phosphodiesterase activities were constrained to approximate values measured in pulmonary microvascular endothelial cells. These simulations suggest that the subcellular localizations of adenylyl cyclase and phosphodiesterase activities are by themselves insufficient to generate physiologically relevant cAMP gradients. Thus, the assembly of adenylyl cyclase, phosphodiesterase, and protein kinase A onto protein scaffolds is by itself unlikely to ensure signal specificity. Rather, our simulations suggest that reductions in the effective cAMP diffusion coefficient may facilitate the formation of substantial cAMP gradients. We conclude that reductions in the effective rate of cAMP diffusion due to buffers, structural impediments, and local changes in viscosity greatly facilitate the ability of signaling complexes to impart specificity within the cAMP signaling pathway.
Figures
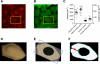
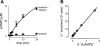

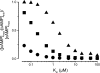

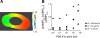
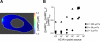

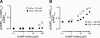


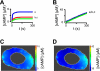
Comment in
-
The interplay of multiple molecular and cellular components is necessary for compartmentalization of cAMP. Focus on "Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells".Am J Physiol Cell Physiol. 2012 Mar 15;302(6):C837-8. doi: 10.1152/ajpcell.00012.2012. Epub 2012 Jan 11. Am J Physiol Cell Physiol. 2012. PMID: 22237404 No abstract available.
Similar articles
-
Filamin A is a phosphorylation target of membrane but not cytosolic adenylyl cyclase activity.Am J Physiol Lung Cell Mol Physiol. 2011 Jul;301(1):L117-24. doi: 10.1152/ajplung.00417.2009. Epub 2011 Apr 8. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21478251 Free PMC article.
-
Roles of GRK and PDE4 activities in the regulation of beta2 adrenergic signaling.J Gen Physiol. 2008 Apr;131(4):349-64. doi: 10.1085/jgp.200709881. Epub 2008 Mar 17. J Gen Physiol. 2008. PMID: 18347080 Free PMC article.
-
cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes.Mol Pharmacol. 2018 Apr;93(4):270-276. doi: 10.1124/mol.117.110825. Epub 2017 Dec 7. Mol Pharmacol. 2018. PMID: 29217670 Free PMC article. Review.
-
Compartmentalization of adenylate cyclase and cAMP signalling.Biochem Soc Trans. 2005 Dec;33(Pt 6):1319-22. doi: 10.1042/BST0331319. Biochem Soc Trans. 2005. PMID: 16246108 Review.
-
The interplay of multiple molecular and cellular components is necessary for compartmentalization of cAMP. Focus on "Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells".Am J Physiol Cell Physiol. 2012 Mar 15;302(6):C837-8. doi: 10.1152/ajpcell.00012.2012. Epub 2012 Jan 11. Am J Physiol Cell Physiol. 2012. PMID: 22237404 No abstract available.
Cited by
-
A three-dimensional finite element model of cAMP signals.Forces Mech. 2021 Oct;4:100041. doi: 10.1016/j.finmec.2021.100041. Epub 2021 Sep 4. Forces Mech. 2021. PMID: 35072121 Free PMC article.
-
Perspectives on: Cyclic nucleotide microdomains and signaling specificity.J Gen Physiol. 2014 Jan;143(1):5-7. doi: 10.1085/jgp.201311144. J Gen Physiol. 2014. PMID: 24378902 Free PMC article. No abstract available.
-
Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis.J Biol Chem. 2019 Jan 25;294(4):1095-1103. doi: 10.1074/jbc.AC118.004905. Epub 2018 Dec 17. J Biol Chem. 2019. PMID: 30559293 Free PMC article.
-
Compartmentalized cAMP Signaling Associated With Lipid Raft and Non-raft Membrane Domains in Adult Ventricular Myocytes.Front Pharmacol. 2018 Apr 23;9:332. doi: 10.3389/fphar.2018.00332. eCollection 2018. Front Pharmacol. 2018. PMID: 29740315 Free PMC article.
-
A two-dimensional finite element model of cyclic adenosine monophosphate (cAMP) intracellular signaling.SN Appl Sci. 2019 Dec;1(12):1713. doi: 10.1007/s42452-019-1757-9. Epub 2019 Nov 28. SN Appl Sci. 2019. PMID: 33615142 Free PMC article.
References
-
- Abi-Gerges A, Richter W, Lefebvre F, Mateo P, Varin A, Heymes C, Samuel JL, Lugnier C, Conti M, Fischmeister R, Vandecasteele G. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on beta-adrenergic cAMP signals. Circ Res 105: 784–792, 2009 - PMC - PubMed
-
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815, 1992 - PubMed
-
- Anderson RG, Jacobson K. a role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296: 1821–1825, 2002 - PubMed
-
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, Jin SL, Conti M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol 173: 7531–7538, 2004 - PubMed
-
- Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+. Am J Physiol Heart Circ Physiol 279: H679–H691, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

