STAS domain structure and function
- PMID: 22116355
- PMCID: PMC3709189
- DOI: 10.1159/000335104
STAS domain structure and function
Abstract
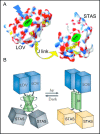
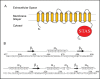
Pendrin shares with nearly all SLC26/SulP anion transporters a carboxy-terminal cytoplasmic segment organized around a Sulfate Transporter and Anti-Sigma factor antagonist (STAS) domain. STAS domains of divergent amino acid sequence exhibit a conserved fold of 4 β strands interspersed among 5 α helices. The first STAS domain proteins studied were single-domain anti-sigma factor antagonists (anti-anti-σ). These anti-anti-σ indirectly stimulate bacterial RNA polymerase by inactivating inhibitory anti-σ kinases, liberating σ factors to direct specific transcription of target genes or operons. Some STAS domains are nucleotide-binding phosphoproteins or nucleotidases. Others are interaction/transduction modules within multidomain sensors of light, oxygen and other gasotransmitters, cyclic nucleotides, inositol phosphates, and G proteins. Additional multidomain STAS protein sequences suggest functions in sensing, metabolism, or transport of nutrients such as sugars, amino acids, lipids, anions, vitamins, or hydrocarbons. Still other multidomain STAS polypeptides include histidine and serine/threonine kinase domains and ligand-activated transcription factor domains. SulP/SLC26 STAS domains and adjacent sequences interact with other transporters, cytoskeletal scaffolds, and with enzymes metabolizing transported anion substrates, forming putative metabolons. STAS domains are central to membrane targeting of many SulP/SLC26 anion transporters, and STAS domain mutations are associated with at least three human recessive diseases. This review summarizes STAS domain structure and function.
Copyright © 2011 S. Karger AG, Basel.
Figures







Similar articles
-
Molecular dynamics simulations of the STAS domains of rat prestin and human pendrin reveal conformational motions in conserved flexible regions.Cell Physiol Biochem. 2014;33(3):605-20. doi: 10.1159/000358638. Epub 2014 Feb 27. Cell Physiol Biochem. 2014. PMID: 24603188
-
Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters.J Mol Biol. 2010 Jul 16;400(3):448-62. doi: 10.1016/j.jmb.2010.05.013. Epub 2010 May 21. J Mol Biol. 2010. PMID: 20471983
-
Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis.J Biol Chem. 2011 Mar 11;286(10):8534-8544. doi: 10.1074/jbc.M110.165449. Epub 2010 Dec 29. J Biol Chem. 2011. PMID: 21190940 Free PMC article.
-
STAS Domain Only Proteins in Bacterial Gene Regulation.Front Cell Infect Microbiol. 2021 Jun 21;11:679982. doi: 10.3389/fcimb.2021.679982. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34235094 Free PMC article. Review.
-
The SLC26 gene family of anion transporters and channels.Mol Aspects Med. 2013 Apr-Jun;34(2-3):494-515. doi: 10.1016/j.mam.2012.07.009. Mol Aspects Med. 2013. PMID: 23506885 Free PMC article. Review.
Cited by
-
Functional variants of human papillomavirus type 16 demonstrate host genome integration and transcriptional alterations corresponding to their unique cancer epidemiology.BMC Genomics. 2016 Nov 2;17(1):851. doi: 10.1186/s12864-016-3203-3. BMC Genomics. 2016. PMID: 27806689 Free PMC article.
-
CFTR-SLC26 transporter interactions in epithelia.Biophys Rev. 2012 Jun 1;4(2):107-116. doi: 10.1007/s12551-012-0068-9. Epub 2012 Feb 15. Biophys Rev. 2012. PMID: 22685498 Free PMC article.
-
Chemotaxis Coupling Protein CheW2 Is Not Required for the Chemotaxis but Contributes to the Full Pathogenicity of Borreliella burgdorferi.Infect Immun. 2023 Apr 18;91(4):e0000823. doi: 10.1128/iai.00008-23. Epub 2023 Mar 20. Infect Immun. 2023. PMID: 36939335 Free PMC article.
-
The discovery of novel noncoding RNAs in 50 bacterial genomes.Nucleic Acids Res. 2024 May 22;52(9):5152-5165. doi: 10.1093/nar/gkae248. Nucleic Acids Res. 2024. PMID: 38647067 Free PMC article.
-
SLC26 Cl-/HCO3- exchangers in the kidney: roles in health and disease.Kidney Int. 2013 Oct;84(4):657-66. doi: 10.1038/ki.2013.138. Epub 2013 May 1. Kidney Int. 2013. PMID: 23636174 Free PMC article. Review.
References
-
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. - PubMed
-
- Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. - PubMed
-
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. - PubMed
-
- Kere J. Overview of the SLC26 family and associated diseases. Novartis Found Symp. 2006;273:2–11. disc. 11-18, 261-264. - PubMed
-
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology. 2008;23:104–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
