The (not necessarily) convoluted role of basal radial glia in cortical neurogenesis
- PMID: 22116731
- PMCID: PMC3256413
- DOI: 10.1093/cercor/bhr336
The (not necessarily) convoluted role of basal radial glia in cortical neurogenesis
Abstract
Recent advances in cell labeling and imaging techniques have dramatically expanded our knowledge of the neural precursor cells responsible for corticogenesis. In particular, radial glial cells are now known to generate several classes of restricted progenitors and neurons. While radial glial cells in the ventricular zone have received the most attention, it has become increasingly clear that a distinct subclass of radial glial cells situated in the subventricular zone (SVZ) and intermediate zone also play an important role in corticogenesis. These delaminated radial glial cells, which lack an apical process attached to the ventricular surface but maintain a basal process, were discovered over 3 decades ago. Recently, they have been further characterized as cortical progenitors and renamed outer, intermediate, or basal radial glia (bRG). Some of these studies indicated that bRG abundance in the outer SVZ (oSVZ) is correlated with enhanced gyrencephaly, particularly in primates and especially human, and therefore suggested that bRG may be responsible for the emergence and evolution of cerebral convolutions. In this issue of Cerebral Cortex, 2 papers provide new information about bRG in common marmosets, a near-lissencephalic primate, and in agouti, a near-gyrencephalic rodent (Garcia-Moreno et al. 2011; Kelava et al. 2011). They demonstrate that bRG are abundant and proliferate in inner as well as oSVZ, in both species. Together, these findings indicate that bRG and the oSVZ might not be correlated with gyrification or phylogeny. Rather, differential regulation of bRG and other progenitor types may enhance the adaptability and diversity of cortical morphogenesis.
Figures
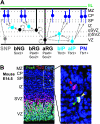

Comment on
-
Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent.Cereb Cortex. 2012 Feb;22(2):482-92. doi: 10.1093/cercor/bhr312. Epub 2011 Nov 23. Cereb Cortex. 2012. PMID: 22114081
-
Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus.Cereb Cortex. 2012 Feb;22(2):469-81. doi: 10.1093/cercor/bhr301. Epub 2011 Nov 23. Cereb Cortex. 2012. PMID: 22114084 Free PMC article.
References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Bystron I, Blakemore C, Rakic P. Development of human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. - PubMed
-
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. - PubMed
-
- Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. - PubMed

