Molecular Mechanics Investigation of an Adenine-Adenine Non-Canonical Pair Conformational Change
- PMID: 22116780
- PMCID: PMC3221466
- DOI: 10.1021/ct200223q
Molecular Mechanics Investigation of an Adenine-Adenine Non-Canonical Pair Conformational Change
Abstract
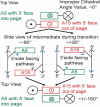
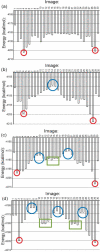
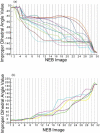
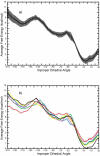
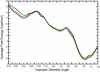
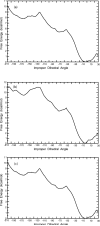
Conformational changes are important in RNA for binding and catalysis and understanding these changes is important for understanding how RNA functions. Computational techniques using all-atom molecular models can be used to characterize conformational changes in RNA. These techniques are applied to an RNA conformational change involving a single base pair within a nine base pair RNA duplex. The Adenine-Adenine (AA) non-canonical pair in the sequence 5'GGUGAAGGCU3' paired with 3'PCCGAAGCCG5', where P is Purine, undergoes conformational exchange between two conformations on the timescale of tens of microseconds, as demonstrated in a previous NMR solution structure [Chen, G., et al., Biochemistry, 2006. 45: 6889-903]. The more populated, major, conformation was estimated to be 0.5 to 1.3 kcal/mol more stable at 30 °C than the less populated, minor, conformation. Both conformations are trans-Hoogsteen/sugar edge pairs, where the interacting edges on the adenines change with the conformational change. Targeted Molecular Dynamics (TMD) and Nudged Elastic Band (NEB) were used to model the pathway between the major and minor conformations using the AMBER software package. The adenines were predicted to change conformation via intermediates in which they are stacked as opposed to hydrogen-bonded. The predicted pathways can be described by an improper dihedral angle reaction coordinate. Umbrella sampling along the reaction coordinate was performed to model the free energy profile for the conformational change using a total of 1800 ns of sampling. Although the barrier height between the major and minor conformations was reasonable, the free energy difference between the major and minor conformations was the opposite of that expected based on the NMR experiments. Variations in the force field applied did not improve the misrepresentation of the free energies of the major and minor conformations. As an alternative, the Molecular Mechanics Poisson-Boltzmann Surface Area (MMPBSA) approximation was applied to predict free energy differences between the two conformations using a total of 800 ns of sampling. MM-PBSA also incorrectly predicted the major conformation to be higher in free energy than the minor conformation.
Figures



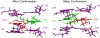
References
-
- Williamson JR. Induced fit in RNA-protein recognition. Nat. Struct. Mol. Biol. 2000;7:834–837. - PubMed
-
- Patel DJ. Adaptive recognition in RNA complexes with peptides and protein modules. Curr. Opin. Struct. Biol. 1999;9:74–87. - PubMed
-
- Leulliot N, Varani G. Current topics in RNA-protein recognition: Control of specificity and biological function through induced fit and conformational capture. Biochemistry. 2001;40:7947–7956. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
