Macrolide antibiotics and survival in patients with acute lung injury
- PMID: 22116799
- PMCID: PMC3342785
- DOI: 10.1378/chest.11-1908
Macrolide antibiotics and survival in patients with acute lung injury
Abstract
Background: Animal models suggest that immunomodulatory properties of macrolide antibiotics have therapeutic value for patients with acute lung injury (ALI). We investigated the association between receipt of macrolide antibiotics and clinical outcomes in patients with ALI.
Methods: Secondary analysis of multicenter, randomized controlled trial data from the Acute Respiratory Distress Syndrome Network Lisofylline and Respiratory Management of Acute Lung Injury Trial, which collected detailed data regarding antibiotic use among participants with ALI.
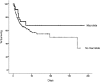
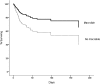
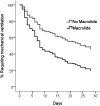
Results: Forty-seven of 235 participants (20%) received a macrolide antibiotic within 24 h of trial enrollment. Among patients who received a macrolide, erythromycin was the most common (57%), followed by azithromycin (40%). The median duration of macrolide use after study enrollment was 4 days (interquartile range, 2-8 days). Eleven of the 47 (23%) patients who received macrolides died, compared with 67 of the 188 (36%) who did not receive a macrolide (P = .11). Participants administered macrolides were more likely to have pneumonia as an ALI risk factor, were less likely to have nonpulmonary sepsis or to be randomized to low tidal volume ventilation, and had a shorter length of stay prior to trial enrollment. After adjusting for potentially confounding covariates, use of macrolide was associated with lower 180-day mortality (hazard ratio [HR], 0.46; 95% CI, 0.23-0.92; P = .028) and shorter time to successful discontinuation of mechanical ventilation (HR, 1.93; 95% CI, 1.18-3.17; P = .009). In contrast, fluoroquinolone (n = 90) and cephalosporin antibiotics (n = 93) were not associated with improved outcomes.
Conclusions: Receipt of macrolide antibiotics was associated with improved outcomes in patients with ALI.
Figures
Comment in
-
Macrolides for acute lung injury.Chest. 2012 May;141(5):1131-1132. doi: 10.1378/chest.11-3245. Chest. 2012. PMID: 22553255 Free PMC article. No abstract available.
-
24/7 intensivist coverage, macrolides in acute lung injury, and roflumilast.Am J Respir Crit Care Med. 2013 Feb 15;187(4):446-7. doi: 10.1164/rccm.201208-1528RR. Am J Respir Crit Care Med. 2013. PMID: 23418328 No abstract available.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. - PubMed
-
- The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. - PubMed
-
- The ARDS Network Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283(15):1995–2002. - PubMed
-
- Steinberg KP, Hudson LD, Goodman RB, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

