Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat
- PMID: 22116802
- PMCID: PMC3316269
- DOI: 10.1095/biolreprod.111.095588
Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat
Abstract
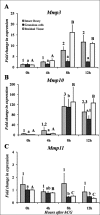
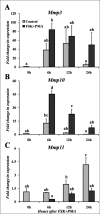
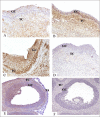
The matrix metalloproteinases (MMPs) are postulated to facilitate follicular rupture. In the present study, expression of the stromelysins (MMP3, MMP10, MMP11) was analyzed in the periovulatory human and rat ovary. Human granulosa and theca cells were collected from the dominant follicle at various times after human chorionic gonadotropin (hCG). Intact rat ovaries, granulosa cells, and residual tissue (tissue remaining after granulosa cell collection) were isolated from equine CG (eCG)-hCG-primed animals. Mmp10 mRNA was highly induced in human granulosa and theca cells and intact rat ovaries, granulosa cells, and residual tissue. Localization of MMP10 to granulosa and theca cells in both human and rat ovarian follicles was confirmed by immunohistochemistry. Mmp3 mRNA was unchanged in human cells and rat granulosa cells, but increased in intact rat ovaries and residual tissue. Mmp11 mRNA decreased following hCG treatment in human granulosa and theca cells as well as rat granulosa cells. Regulation of Mmp10 in cultured rat granulosa cells revealed that the EGF inhibitor AG1478 and the progesterone receptor antagonist RU486 suppressed the induction of Mmp10 mRNA, whereas the prostaglandin inhibitor NS398 had no effect. Studies on the Mmp10 promoter demonstrated that forskolin plus PMA stimulated promoter activity, which was dependent upon a proximal AP1 site. In conclusion, there are divergent patterns of stromelysin expression associated with ovulation, with a marked induction of Mmp10 mRNA and a decrease in Mmp11 mRNA, yet a species-dependent pattern on Mmp3 mRNA expression. The induction of Mmp10 expression suggests an important role for this MMP in the follicular changes associated with ovulation and subsequent luteinization.
Figures




References
-
- Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med 2006; 24: 228 241 - PubMed
-
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428 465 - PubMed
-
- Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta 2005; 1751: 95 109 - PubMed
-
- Goldman S, Shalev E. MMPs and TIMPS in ovarian physiology and pathophysiology. Front Biosci 2004; 9: 2474 2483 - PubMed
-
- Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res 2003; 44: 50 57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

