Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity
- PMID: 22116882
- PMCID: PMC3266580
- DOI: 10.1126/science.1213920
Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity
Abstract
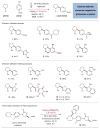
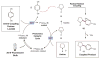
Serendipity has long been a welcome yet elusive phenomenon in the advancement of chemistry. We sought to exploit serendipity as a means of rapidly identifying unanticipated chemical transformations. By using a high-throughput, automated workflow and evaluating a large number of random reactions, we have discovered a photoredox-catalyzed C-H arylation reaction for the construction of benzylic amines, an important structural motif within pharmaceutical compounds that is not readily accessed via simple substrates. The mechanism directly couples tertiary amines with cyanoaromatics by using mild and operationally trivial conditions.
Figures



References
-
- Roberts RM. Serendipity: Accidental Discoveries in Science. Wiley; New York: 1989.
-
-
Other examples of reaction discovery using a combinatorial approach have been reported (–11).
-
-
- Weber L, Illgen K, Almstetter M. Synlett. 1999;1999:366.
-
- Beeler AB, Su S, Singleton CA, Porco JA., Jr J Am Chem Soc. 2007;129:1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

