The role of catalase in gonococcal resistance to peroxynitrite
- PMID: 22117004
- PMCID: PMC3352282
- DOI: 10.1099/mic.0.053686-0
The role of catalase in gonococcal resistance to peroxynitrite
Abstract
We have reported that Neisseria gonorrhoeae is extremely resistant to reactive nitrogen species (RNS) including peroxynitrite (PN). Recent literature suggests that catalase can provide protection against commercial preparations of PN. Though wild-type gonococci were shown to be highly resistant to 2 mM PN, Neisseria meningitidis and a gonococcal katA mutant were both shown to be extremely sensitive to 2 mM PN. Analysis of translational fusions to lacZ of the catalase promoters from N. gonorrhoeae and N. meningitidis demonstrated that basal katA expression from gonococci is 80-fold higher than in meningococci, though meningococcal katA retains a greater capacity to be activated by OxyR. This activation capacity was shown to be due to a single base pair difference in the -10 transcription element between the two kat promoters. PN resistance was initially shown to be associated with increasing catalase expression; however, commercial preparations of PN were later revealed to contain higher levels of contaminating hydrogen peroxide (H2O2) than expected. Removal of H2O2 from PN preparations with manganese dioxide markedly reduced PN toxicity in a gonococcal katA mutant. Simultaneous treatment with non-lethal concentrations of PN and H2O2 was highly lethal, indicating that these agents act synergistically. When treatment was separated by 5 min, high levels of bacterial killing occurred only when PN was added first. Our results suggest that killing of N. gonorrhoeae ΔkatA by commercial PN preparations is likely due to H2O2, that H2O2 is more toxic in the presence of PN, and that PN, on its own, may not be as toxic as previously believed.
Figures


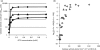

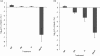

Similar articles
-
Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response.Infect Immun. 2007 May;75(5):2225-33. doi: 10.1128/IAI.01513-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296753 Free PMC article.
-
A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals.Microb Pathog. 2004 Aug;37(2):55-63. doi: 10.1016/j.micpath.2004.04.007. Microb Pathog. 2004. PMID: 15312845
-
OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae.Infect Immun. 2003 Jan;71(1):550-6. doi: 10.1128/IAI.71.1.550-556.2003. Infect Immun. 2003. PMID: 12496210 Free PMC article.
-
Drug-resistant Neisseria gonorrhoeae: latest developments.Eur J Clin Microbiol Infect Dis. 2017 Jul;36(7):1065-1071. doi: 10.1007/s10096-017-2931-x. Epub 2017 Feb 16. Eur J Clin Microbiol Infect Dis. 2017. PMID: 28210887 Review.
-
Emerging resistance in Neisseria meningitidis and Neisseria gonorrhoeae.Expert Rev Anti Infect Ther. 2011 Feb;9(2):237-44. doi: 10.1586/eri.10.171. Expert Rev Anti Infect Ther. 2011. Retraction in: Expert Rev Anti Infect Ther. 2011 Dec;9(12):1204. doi: 10.1586/eri.11.146. PMID: 21342071 Retracted. Review.
Cited by
-
How the Knowledge of Interactions between Meningococcus and the Human Immune System Has Been Used to Prepare Effective Neisseria meningitidis Vaccines.J Immunol Res. 2015;2015:189153. doi: 10.1155/2015/189153. Epub 2015 Aug 17. J Immunol Res. 2015. PMID: 26351643 Free PMC article. Review.
-
Bacterium of one thousand and one variants: genetic diversity of Neisseria gonorrhoeae pathogenicity.Microb Genom. 2023 Jun;9(6):mgen001040. doi: 10.1099/mgen.0.001040. Microb Genom. 2023. PMID: 37285200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

