Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia
- PMID: 22117041
- PMCID: PMC3286344
- DOI: 10.1182/blood-2011-09-381731
Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia
Abstract
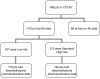
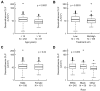
We have previously hypothesized that higher systemic exposure to asparaginase may cause increased exposure to dexamethasone, both critical chemotherapeutic agents for acute lymphoblastic leukemia. Whether interpatient pharmaco-kinetic differences in dexamethasone contribute to relapse risk has never been studied. The impact of plasma clearance of dexamethasone and anti-asparaginase antibody levels on risk of relapse was assessed in 410 children who were treated on a front-line clinical trial for acute lymphoblastic leukemia and were evaluable for all pharmacologic measures, using multivariate analyses, adjusting for standard clinical and biologic prognostic factors. Dexamethasone clearance (mean ± SD) was higher (P = 3 × 10(-8)) in patients whose sera was positive (17.7 ± 18.6 L/h per m(2)) versus nega-tive (10.6 ± 5.99 L/h per m(2)) for anti-asparaginase antibodies. In multivariate analyses, higher dexamethasone clearance was associated with a higher risk of any relapse (P = .01) and of central nervous system relapse (P = .014). Central nervous system relapse was also more common in patients with anti-asparaginase antibodies (P = .019). In conclusion, systemic clearance of dexamethasone is higher in patients with anti-asparaginase antibodies. Lower exposure to both drugs was associated with an increased risk of relapse.
Figures
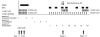


Comment in
-
The right dose for the right patient.Blood. 2012 Feb 16;119(7):1617-8. doi: 10.1182/blood-2011-12-395855. Blood. 2012. PMID: 22343660 No abstract available.
References
-
- Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101(10):3809–3817. - PubMed
-
- Jones B, Freeman AI, Shuster JJ, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19:269–275. - PubMed
-
- Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–1939. - PubMed
-
- Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18(7):1525–1532. - PubMed
-
- Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26(4):217–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

