Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin
- PMID: 22117063
- PMCID: PMC3256866
- DOI: 10.1074/jbc.M111.297150
Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin
Abstract
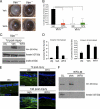
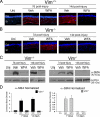
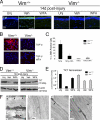
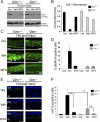
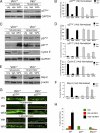
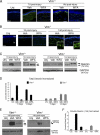
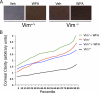
The type III intermediate filaments (IFs) are essential cytoskeletal elements of mechanosignal transduction and serve critical roles in tissue repair. Mice genetically deficient for the IF protein vimentin (Vim(-/-)) have impaired wound healing from deficits in myofibroblast development. We report a surprising finding made in Vim(-/-) mice that corneas are protected from fibrosis and instead promote regenerative healing after traumatic alkali injury. This reparative phenotype in Vim(-/-) corneas is strikingly recapitulated by the pharmacological agent withaferin A (WFA), a small molecule that binds to vimentin and down-regulates its injury-induced expression. Attenuation of corneal fibrosis by WFA is mediated by down-regulation of ubiquitin-conjugating E3 ligase Skp2 and up-regulation of cyclin-dependent kinase inhibitors p27(Kip1) and p21(Cip1). In cell culture models, WFA exerts G(2)/M cell cycle arrest in a p27(Kip1)- and Skp2-dependent manner. Finally, by developing a highly sensitive imaging method to measure corneal opacity, we identify a novel role for desmin overexpression in corneal haze. We demonstrate that desmin down-regulation by WFA via targeting the conserved WFA-ligand binding site shared among type III IFs promotes further improvement of corneal transparency without affecting cyclin-dependent kinase inhibitor levels in Vim(-/-) mice. This dissociates a direct role for desmin in corneal cell proliferation. Taken together, our findings illuminate a previously unappreciated pathogenic role for type III IF overexpression in corneal fibrotic conditions and also validate WFA as a powerful drug lead toward anti-fibrosis therapeutic development.
Figures

References
-
- Taneri S., Weisberg M., Azar D. T. (2011) Surface ablation techniques. J. Cataract Refract. Surg. 37, 392–408 - PubMed
-
- Schaffeld M., Herrmann H., Schultess J., Markl J. (2001) Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris. Sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 80, 692–702 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

