Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21(Cip1) expression and hampers tumour cell growth in vitro and in vivo
- PMID: 22117196
- PMCID: PMC3321627
- DOI: 10.1038/cdd.2011.171
Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21(Cip1) expression and hampers tumour cell growth in vitro and in vivo
Abstract
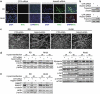
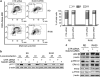
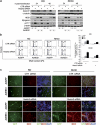
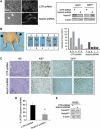
Rhabdomyosarcoma (RMS) is a paediatric soft-tissue sarcoma arising from skeletal muscle precursors coexpressing markers of proliferation and differentiation. Inducers of myogenic differentiation suppress RMS tumourigenic phenotype. The Notch target gene HES1 is upregulated in RMS and prevents tumour cell differentiation in a Notch-dependent manner. However, Notch receptors regulating this phenomenon are unknown. In agreement with data in RMS primary tumours, we show here that the Notch3 receptor is overexpressed in RMS cell lines versus normal myoblasts. Notch3-targeted downregulation in RMS cells induces hyper-phosphorylation of p38 and Akt essential for myogenesis, resulting in the differentiation of tumour cells into multinucleated myotubes expressing Myosin Heavy Chain. These phenomena are associated to a marked decrease in HES1 expression, an increase in p21(Cip1) level and the accumulation of RMS cells in the G1 phase. HES1-forced overexpression in RMS cells reverses, at least in part, the pro-differentiative effects of Notch3 downregulation. Notch3 depletion also reduces the tumourigenic potential of RMS cells both in vitro and in vivo. These results indicate that downregulation of Notch3 is sufficient to force RMS cells into completing a correct full myogenic program providing evidence that it contributes, partially through HES1 sustained expression, to their malignant phenotype. Moreover, they suggest Notch3 as a novel potential target in human RMS.
Figures



References
-
- Williamson D, Missiaglia E, de Reynies A, Pierron G, Thuille B, Palenzuela G, et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J Clin Oncol. 2010;28:2151–2158. - PubMed
-
- Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–1453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

