The monarch butterfly genome yields insights into long-distance migration
- PMID: 22118469
- PMCID: PMC3225893
- DOI: 10.1016/j.cell.2011.09.052
The monarch butterfly genome yields insights into long-distance migration
Abstract
We present the draft 273 Mb genome of the migratory monarch butterfly (Danaus plexippus) and a set of 16,866 protein-coding genes. Orthology properties suggest that the Lepidoptera are the fastest evolving insect order yet examined. Compared to the silkmoth Bombyx mori, the monarch genome shares prominent similarity in orthology content, microsynteny, and protein family sizes. The monarch genome reveals a vertebrate-like opsin whose existence in insects is widespread; a full repertoire of molecular components for the monarch circadian clockwork; all members of the juvenile hormone biosynthetic pathway whose regulation shows unexpected sexual dimorphism; additional molecular signatures of oriented flight behavior; microRNAs that are differentially expressed between summer and migratory butterflies; monarch-specific expansions of chemoreceptors potentially important for long-distance migration; and a variant of the sodium/potassium pump that underlies a valuable chemical defense mechanism. The monarch genome enhances our ability to better understand the genetic and molecular basis of long-distance migration.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

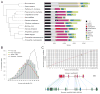

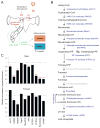
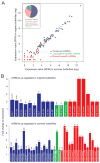

Comment in
-
A genome befitting a monarch.Cell. 2011 Nov 23;147(5):970-2. doi: 10.1016/j.cell.2011.11.009. Cell. 2011. PMID: 22118454
References
-
- Baumann O, Salvaterra PM, Takeyasu K. Developmental changes in beta-subunit composition of Na,K-ATPase in the Drosophila eye. Cell Tissue Res. 2010;340:215–228. - PubMed
-
- Belles X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 2005;50:181–199. - PubMed
-
- Benna C, Bonaccorsi S, Wulbeck C, Helfrich-Forster C, Gatti M, Kyriacou CP, Costa R, Sandrelli F. Drosophila timeless2 is required for chromosome stability and circadian photoreception. Curr Biol. 2010;20:346–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

