Clinical and virologic outcomes in patients with oseltamivir-resistant seasonal influenza A (H1N1) infections: results from a clinical trial
- PMID: 22118629
- PMCID: PMC4941666
- DOI: 10.1111/j.1750-2659.2011.00312.x
Clinical and virologic outcomes in patients with oseltamivir-resistant seasonal influenza A (H1N1) infections: results from a clinical trial
Abstract
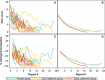
Nineteen patients with oseltamivir-resistant seasonal influenza A (H1N1) infections were randomized to receive oseltamivir or placebo. Nasopharyngeal swabs were obtained, and clinical and virologic outcomes were compared, stratified by early or late treatment. Neuraminidase inhibition assay and pyrosequencing for H275Y confirmed resistance. Twelve (63%) patients received oseltamivir; 8 (67%) received late treatment. Seven (37%) patients received placebo; 6 (86%) presented >48 hours after onset. Time to 50% decrease in symptom severity, complete symptom resolution, and first negative culture were shortest among the early treatment group. While sample size prohibits a strong conclusion, future studies should evaluate for similar trends.
© 2011 Blackwell Publishing Ltd.
Figures

References
-
- Belongia EA, Kieke BA, Donahue JG et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004‐2005 season to the 2006–2007 season. J Infect Dis 2009; 199:159–167. - PubMed
-
- Deyde VM, Okomo‐Adhiambo M, Sheu TG et al. Pyrosequencing as a tool to detect molecular markers of resistance to neuraminidase inhibitors in seasonal influenza A viruses. Antiviral Res 2009; 81:16–24. - PubMed
-
- Eilers PHC, Marx BD. Flexible smoothing with B‐splines and penalties. Statistical Science 1996; 11:89–121, with discussion.
-
- Kawai N, Ikematsu H, Hirotsu N et al. Clinical effectiveness of oseltamivir and zanamivir for treatment of influenza A virus subtype H1N1 with the H274Y mutation: a Japanese, multicenter study of the 2007–2008 and 2008–2009 influenza seasons. Clin Infect Dis 2009; 49:1828–1835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

