Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans
- PMID: 22119442
- PMCID: PMC3250924
- DOI: 10.1016/j.cell.2011.10.042
Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans
Abstract
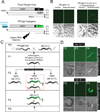
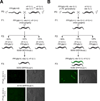
Induced expression of the Flock House virus in the soma of C. elegans results in the RNAi-dependent production of virus-derived, small-interfering RNAs (viRNAs), which in turn silence the viral genome. We show here that the viRNA-mediated viral silencing effect is transmitted in a non-Mendelian manner to many ensuing generations. We show that the viral silencing agents, viRNAs, are transgenerationally transmitted in a template-independent manner and work in trans to silence viral genomes present in animals that are deficient in producing their own viRNAs. These results provide evidence for the transgenerational inheritance of an acquired trait, induced by the exposure of animals to a specific, biologically relevant physiological challenge. The ability to inherit such extragenic information may provide adaptive benefits to an animal.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Ball LA, Wohlrab B, Li Y. Nodavirus RNA replication: mechanism and harnessing to vaccinia virus recombinants. Arch Virol Suppl. 1994;9:407–416. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

