Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy
- PMID: 22119795
- PMCID: PMC3299818
- DOI: 10.1016/j.mito.2011.11.002
Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy
Abstract
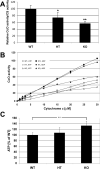
Subunit 7a of mouse cytochrome c oxidase (Cox) displays a contractile muscle-specific isoform, Cox7a1, that is the major cardiac form. To gain insight into the role of this isoform, we have produced a new knockout mouse line that lacks Cox7a1. We show that homozygous and heterozygous Cox7a1 knockout mice, although viable, have reduced Cox activity and develop a dilated cardiomyopathy at 6 weeks of age. Surprisingly, the cardiomyopathy improves and stabilizes by 6 months of age. Cox7a1 knockout mice incorporate more of the "liver-type" isoform Cox7a2 into the cardiac Cox holoenzyme and, also surprisingly, have higher tissue ATP levels.
Copyright © 2011 Elsevier B.V. and Mitochondria Research Society. All rights reserved. All rights reserved.
Figures





References
-
- Acin-Perez R, Bayona-Bafaluy MP, Bueno M, Machicado C, Fernandez-Silva P, Perez-Martos A, Montoya J, Lopez-Perez MJ, Sancho J, Enriquez JA. An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum. Mol. Genet. 2003;12(3):329–339. - PubMed
-
- Arber S, Hunter JJ, Ross J, Jr., Hongo M, Sansig G, Borg J, Perriard J-C, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88(3):393–403. - PubMed
-
- Bonne G, Seibel P, Possekel S, Marsac C, Kadenbach B. Expression of human cytochrome-c oxidase subunits during fetal development. Eur. J. Biochem. 1993;217(3):1099–1107. - PubMed
-
- Casali C, Damati G, Bernucci P, Debiase L, Autore C, Santorelli FM, Coviello D, Gallo P. Maternally inherited cardiomyopathy: Clinical and molecular characterization of a large kindred harboring the A4300G point mutation in mitochondrial deoxyribonucleic acid. J. Amer. Coll. Cardiol. 1999;33(6):1584–1589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

