Diffusion MRI at 25: exploring brain tissue structure and function
- PMID: 22120012
- PMCID: PMC3683822
- DOI: 10.1016/j.neuroimage.2011.11.006
Diffusion MRI at 25: exploring brain tissue structure and function
Abstract
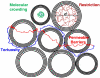
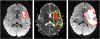
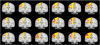
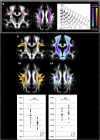
Diffusion MRI (or dMRI) came into existence in the mid-1980s. During the last 25 years, diffusion MRI has been extraordinarily successful (with more than 300,000 entries on Google Scholar for diffusion MRI). Its main clinical domain of application has been neurological disorders, especially for the management of patients with acute stroke. It is also rapidly becoming a standard for white matter disorders, as diffusion tensor imaging (DTI) can reveal abnormalities in white matter fiber structure and provide outstanding maps of brain connectivity. The ability to visualize anatomical connections between different parts of the brain, non-invasively and on an individual basis, has emerged as a major breakthrough for neurosciences. The driving force of dMRI is to monitor microscopic, natural displacements of water molecules that occur in brain tissues as part of the physical diffusion process. Water molecules are thus used as a probe that can reveal microscopic details about tissue architecture, either normal or in a diseased state.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319. - PubMed
-
- Anderson AW, Gore JC. Analysis and correction of motion arifacts in diffusion weighted imaging. Magn. Reson. Med. 1994;32:379–387. - PubMed
-
- Andrew RD, Macvicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. - PubMed
-
- Anwander A, Tittgemeyer M, Von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca’s area. Cereb. Cortex. 2007;17:816–825. - PubMed
-
- Aso T, Urayama S, Poupon C, Sawamoto N, Fukuyama H, Le Bihan D. An intrinsic diffusion response function for analyzing diffusion functional MRI time series. Neuroimage. 2009;47:1487–1495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

