Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex
- PMID: 22120143
- PMCID: PMC3267981
- DOI: 10.1093/brain/awr303
Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex
Abstract
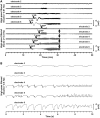
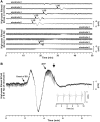
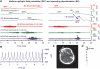
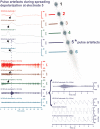
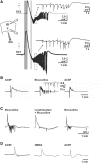
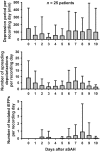
Spreading depolarization of cells in cerebral grey matter is characterized by massive ion translocation, neuronal swelling and large changes in direct current-coupled voltage recording. The near-complete sustained depolarization above the inactivation threshold for action potential generating channels initiates spreading depression of brain activity. In contrast, epileptic seizures show modest ion translocation and sustained depolarization below the inactivation threshold for action potential generating channels. Such modest sustained depolarization allows synchronous, highly frequent neuronal firing; ictal epileptic field potentials being its electrocorticographic and epileptic seizure its clinical correlate. Nevertheless, Leão in 1944 and Van Harreveld and Stamm in 1953 described in animals that silencing of brain activity induced by spreading depolarization changed during minimal electrical stimulations. Eventually, epileptic field potentials were recorded during the period that had originally seen spreading depression of activity. Such spreading convulsions are characterized by epileptic field potentials on the final shoulder of the large slow potential change of spreading depolarization. We here report on such spreading convulsions in monopolar subdural recordings in 2 of 25 consecutive aneurismal subarachnoid haemorrhage patients in vivo and neocortical slices from 12 patients with intractable temporal lobe epilepsy in vitro. The in vitro results suggest that γ-aminobutyric acid-mediated inhibition protects from spreading convulsions. Moreover, we describe arterial pulse artefacts mimicking epileptic field potentials in three patients with subarachnoid haemorrhage that ride on the slow potential peak. Twenty-one of the 25 subarachnoid haemorrhage patients (84%) had 656 spreading depolarizations in contrast to only three patients (12%) with 55 ictal epileptic events isolated from spreading depolarizations. Spreading depolarization frequency and depression periods per 24 h recording episodes showed an early and a delayed peak on Day 7. Patients surviving subarachnoid haemorrhage with poor outcome at 6 months showed significantly higher total and peak numbers of spreading depolarizations and significantly longer total and peak depression periods during the electrocorticographic monitoring than patients with good outcome. In a semi-structured telephone interview 3 years after the initial haemorrhage, 44% of the subarachnoid haemorrhage survivors had developed late post-haemorrhagic seizures requiring anti-convulsant medication. In those patients, peak spreading depolarization number had been significantly higher [15.1 (11.4-30.8) versus 7.0 (0.8-11.2) events per day, P = 0.045]. In summary, monopolar recordings here provided unequivocal evidence of spreading convulsions in patients. Hence, practically all major pathological cortical network events in animals have now been observed in people. Early spreading depolarizations may indicate a risk for late post-haemorrhagic seizures.
Figures
References
-
- Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–96. - PubMed
-
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. - PubMed
-
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–61. - PubMed
-
- Balestrino M, Young J, Aitken P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res. 1999;838:37–44. - PubMed
-
- Berger M, Speckmann EJ, Pape HC, Gorji A. Spreading depression enhances human neocortical excitability in vitro. Cephalalgia. 2008;28:558–62. - PubMed

