Stimulating healthy tissue regeneration by targeting the 5-HT₂B receptor in chronic liver disease
- PMID: 22120177
- PMCID: PMC3428919
- DOI: 10.1038/nm.2490
Stimulating healthy tissue regeneration by targeting the 5-HT₂B receptor in chronic liver disease
Abstract
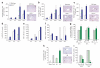
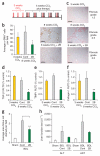
Tissue homeostasis requires an effective, limited wound-healing response to injury. In chronic disease, failure to regenerate parenchymal tissue leads to the replacement of lost cellular mass with a fibrotic matrix. The mechanisms that dictate the balance of cell regeneration and fibrogenesis are not well understood. Here we report that fibrogenic hepatic stellate cells (HSCs) in the liver are negative regulators of hepatocyte regeneration. This negative regulatory function requires stimulation of the 5-hydroxytryptamine 2B receptor (5-HT(2B)) on HSCs by serotonin, which activates expression of transforming growth factor β1 (TGF-β1), a powerful suppressor of hepatocyte proliferation, through signaling by mitogen-activated protein kinase 1 (ERK) and the transcription factor JunD. Selective antagonism of 5-HT(2B) enhanced hepatocyte growth in models of acute and chronic liver injury. We also observed similar effects in mice lacking 5-HT(2B) or JunD or upon selective depletion of HSCs in wild-type mice. Antagonism of 5-HT(2B) attenuated fibrogenesis and improved liver function in disease models in which fibrosis was pre-established and progressive. Pharmacological targeting of 5-HT(2B) is clinically safe in humans and may be therapeutic in chronic liver disease.
Figures
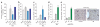

Comment in
-
Activated hepatic stellate cells: negative regulators of hepatocyte proliferation in liver diseases.Hepatology. 2012 Jul;56(1):389-91. doi: 10.1002/hep.25761. Hepatology. 2012. PMID: 22876366 Free PMC article.
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. - PubMed
-
- Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem. J. 2008;411:1–18. - PubMed
-
- Marshall A, et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42. - PubMed
-
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. - PubMed
-
- Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin. Cell Dev. Biol. 2002;13:425–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

