Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance
- PMID: 22120429
- PMCID: PMC3246074
- DOI: 10.1016/j.mam.2011.11.001
Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance
Abstract
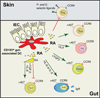
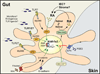
The vitamin A (VA) metabolite all-trans retinoic acid (RA) plays a key role in mucosal immune responses. RA is produced by gut-associated dendritic cells (DC) and is required for generating gut-tropic lymphocytes and IgA-antibody-secreting cells (IgA-ASC). Moreover, RA modulates Foxp3(+) regulatory T cell (T(REG)) and Th17 effector T cell differentiation. Thus, although RA could be used as an effective "mucosal adjuvant" in vaccines, it also appears to be required for establishing intestinal immune tolerance. Here we discuss the roles proposed for RA in shaping intestinal immune responses and tolerance at the gut mucosal interface. We also focus on recent data exploring the mechanisms by which gut-associated DC acquire RA-producing capacity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
References
-
- Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, Lacy-Hulbert A. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. The Journal of clinical investigation. 2010;120(12):4445–4452. - PMC - PubMed
-
- Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. - PubMed
-
- Bhat PV. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett. 1998;426(2):260–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

