Optical recording of action potentials in mammalian neurons using a microbial rhodopsin
- PMID: 22120467
- PMCID: PMC3248630
- DOI: 10.1038/nmeth.1782
Optical recording of action potentials in mammalian neurons using a microbial rhodopsin
Abstract
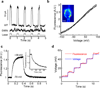
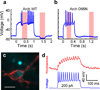
Reliable optical detection of single action potentials in mammalian neurons has been one of the longest-standing challenges in neuroscience. Here we achieved this goal by using the endogenous fluorescence of a microbial rhodopsin protein, Archaerhodopsin 3 (Arch) from Halorubrum sodomense, expressed in cultured rat hippocampal neurons. This genetically encoded voltage indicator exhibited an approximately tenfold improvement in sensitivity and speed over existing protein-based voltage indicators, with a roughly linear twofold increase in brightness between -150 mV and +150 mV and a sub-millisecond response time. Arch detected single electrically triggered action potentials with an optical signal-to-noise ratio >10. Arch(D95N) lacked endogenous proton pumping and had 50% greater sensitivity than wild type but had a slower response (41 ms). Nonetheless, Arch(D95N) also resolved individual action potentials. Microbial rhodopsin-based voltage indicators promise to enable optical interrogation of complex neural circuits and electrophysiology in systems for which electrode-based techniques are challenging.
Figures
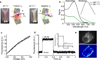


Comment in
-
Running in reverse: rhodopsins sense voltage.Nat Methods. 2011 Dec 28;9(1):43-4. doi: 10.1038/nmeth.1817. Nat Methods. 2011. PMID: 22205516 No abstract available.
References
-
- Cohen LB, Keynes RD, Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968;218:438–441. - PubMed
-
- Akemann W, Mutoh H, Perron A, Rossier J, Knopfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat. Methods. 2010;7:643–649. - PubMed
-
- Scanziani M, Hausser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

