Bilateral and ipsilateral ascending tectopulvinar pathways in mammals: a study in the squirrel (Spermophilus beecheyi)
- PMID: 22120503
- PMCID: PMC3970410
- DOI: 10.1002/cne.23014
Bilateral and ipsilateral ascending tectopulvinar pathways in mammals: a study in the squirrel (Spermophilus beecheyi)
Abstract
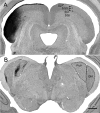
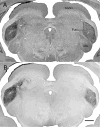
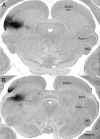
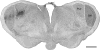
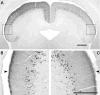
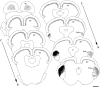
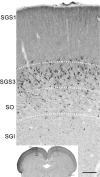
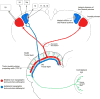
The mammalian pulvinar complex is a collection of dorsal thalamic nuclei related to several visual and integrative processes. Previous studies have shown that the superficial layers of the superior colliculus project to multiple divisions of the pulvinar complex. Although most of these works agree about the existence of an ipsilateral tectopulvinar projection arising from the stratum griseum superficialis, some others report a bilateral projection originating from this same tectal layer. We investigated the organization of the tectopulvinar projections in the Californian ground squirrel using cholera toxin B (CTb). We confirmed previous studies showing that the caudal pulvinar of the squirrel receives a massive bilateral projection originating from a specific cell population located in the superficial collicular layers (SGS3, also called the "lower SGS" or "SGSL"). We found that this projection shares striking structural similarities with the tectorotundal pathway of birds and reptiles. Morphology of the collicular cells originating this projection closely corresponds to that of the bottlebrush tectal cells described previously for chickens and squirrels. In addition, we found that the rostral pulvinar receives an exclusively ipsilateral projection from a spatially separate population of collicular cells located at the base of the stratum opticum, deeper than the cells projecting to the caudal pulvinar. These results strongly support, at a structural level, the homology of the pathway originating in the SGS3 collicular cells upon the caudal pulvinar with the tectorotundal pathway of nonmammalian amniotes and contribute to clarifying the general organization of the tectopulvinar pathways in mammals.
Copyright © 2011 Wiley Periodicals, Inc.
Figures





Similar articles
-
Ultrastructure of ipsilateral and contralateral tectopulvinar projections in the mouse.J Comp Neurol. 2022 May;530(7):1099-1111. doi: 10.1002/cne.25264. Epub 2021 Oct 24. J Comp Neurol. 2022. PMID: 34636423 Free PMC article.
-
Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the mammalian tectum.J Comp Neurol. 2000 Jul 24;423(2):243-60. J Comp Neurol. 2000. PMID: 10867657
-
Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): evidence for four subdivisions within the pulvinar complex.J Comp Neurol. 2011 Apr 15;519(6):1071-94. doi: 10.1002/cne.22552. J Comp Neurol. 2011. PMID: 21344403 Free PMC article.
-
The pulvinar nucleus of Galago senegalensis.J Comp Neurol. 1975 Jun 1;161(3):419-58. doi: 10.1002/cne.901610309. J Comp Neurol. 1975. PMID: 50331
-
The visual superior colliculus and pulvinar.Rev Oculomot Res. 1989;3:337-60. Rev Oculomot Res. 1989. PMID: 2486329 Review.
Cited by
-
The mouse pulvinar nucleus: Organization of the tectorecipient zones.Vis Neurosci. 2017 Jan;34:E011. doi: 10.1017/S0952523817000050. Vis Neurosci. 2017. PMID: 28965504 Free PMC article. Review.
-
Distinguishing externally from saccade-induced motion in visual cortex.Nature. 2022 Oct;610(7930):135-142. doi: 10.1038/s41586-022-05196-w. Epub 2022 Sep 14. Nature. 2022. PMID: 36104560 Free PMC article.
-
Unraveling circuits of visual perception and cognition through the superior colliculus.Neuron. 2021 Mar 17;109(6):918-937. doi: 10.1016/j.neuron.2021.01.013. Epub 2021 Feb 5. Neuron. 2021. PMID: 33548173 Free PMC article. Review.
-
Ultrastructure of ipsilateral and contralateral tectopulvinar projections in the mouse.J Comp Neurol. 2022 May;530(7):1099-1111. doi: 10.1002/cne.25264. Epub 2021 Oct 24. J Comp Neurol. 2022. PMID: 34636423 Free PMC article.
-
Genetically defined neuron types underlying visuomotor transformation in the superior colliculus.Nat Rev Neurosci. 2024 Nov;25(11):726-739. doi: 10.1038/s41583-024-00856-4. Epub 2024 Sep 27. Nat Rev Neurosci. 2024. PMID: 39333418 Review.
References
-
- Aboitiz F. Genetic and developmental homology in amniote brains. Toward conciliating radical views of brain evolution. Brain Res Bull. 2011;84:125–136. - PubMed
-
- Aboitiz F, Montiel J. Origin and evolution of the vertebrate telencephalon, with special reference to the mammalian neocortex. Adv Anat Embryol Cell Biol. 2007;193:1–112. - PubMed
-
- Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26:535–552. discussion 552–585. - PubMed
-
- Abramson BP, Chalupa LM. Multiple pathways from the superior colliculus to the extrageniculate visual thalamus of the cat. J Comp Neurol. 1988;271:397–418. - PubMed
-
- Albano JE, Norton TT, Hall WC. Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Res. 1979;173:1–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

