Regulation of the structure and activity of pyruvate carboxylase by acetyl CoA
- PMID: 22120519
- PMCID: PMC3293938
- DOI: 10.1016/j.abb.2011.11.015
Regulation of the structure and activity of pyruvate carboxylase by acetyl CoA
Abstract
In this review we examine the effects of the allosteric activator, acetyl CoA on both the structure and catalytic activities of pyruvate carboxylase. We describe how the binding of acetyl CoA produces gross changes to the quaternary and tertiary structures of the enzyme that are visible in the electron microscope. These changes serve to stabilize the tetrameric structure of the enzyme. The main locus of activation of the enzyme by acetyl CoA is the biotin carboxylation domain of the enzyme where ATP-cleavage and carboxylation of the biotin prosthetic group occur. As well as enhancing reaction rates, acetyl CoA also enhances the binding of some substrates, especially HCO3-, and there is also a complex interaction with the binding of the cofactor Mg2. The activation of pyruvate carboxylase by acetyl CoA is generally a cooperative processes, although there is a large degree of variability in the degree of cooperativity exhibited by the enzyme from different organisms. The X-ray crystallographic holoenzyme structures of pyruvate carboxylases from Rhizobium etli and Staphylococcus aureus have shown the allosteric acetyl CoA binding domain to be located at the interfaces of the biotin carboxylation and carboxyl transfer and the carboxyl transfer and biotin carboxyl carrier protein domains.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

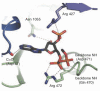




References
-
- Mukhopadhyay B, Patel VJ, Wolfe RS. Arch. Microbiol. 2000;174:406–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

