Interplay between innate and adaptive immunity in the development of non-infectious uveitis
- PMID: 22120610
- PMCID: PMC3288447
- DOI: 10.1016/j.preteyeres.2011.11.004
Interplay between innate and adaptive immunity in the development of non-infectious uveitis
Abstract
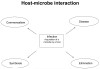
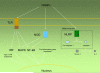
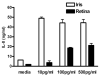
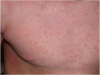
In vertebrates, the innate and adaptive immune systems have evolved seamlessly to protect the host by rapidly responding to danger signals, eliminating pathogens and creating immunological memory as well as immunological tolerance to self. The innate immune system harnesses receptors that recognize conserved pathogen patterns and alongside the more specific recognition systems and memory of adaptive immunity, their interplay is evidenced by respective roles during generation and regulation of immune responses. The hallmark of adaptive immunity which requires engagement of innate immunity is an ability to discriminate between self and non-self (and eventually between pathogen and symbiont) as well as peripheral control mechanisms maintaining immunological health and appropriate responses. Loss of control mechanisms and/or regulation of either the adaptive or the innate immune system lead to autoimmunity and autoinflammation respectively. Although autoimmune pathways have been largely studied to date in the context of development of non-infectious intraocular inflammation, the recruitment and activation of innate immunity is required for full expression of the varied phenotypes of non-infectious uveitis. Since autoimmunity and autoinflammation implicate different molecular pathways, even though some convergence occurs, increasing our understanding of their respective roles in the development of uveitis will highlight treatment targets and influence our understanding of immune mechanisms operative in other retinal diseases. Herein, we extrapolate from the basic mechanisms of activation and control of innate and adaptive immunity to how autoinflammatory and autoimmune pathways contribute to disease development in non-infectious uveitis patients.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abu El-Asrar AM, Abouammoh M, Al-Mezaine HS. Tuberculous uveitis. Int. Ophthalmol. Clin. 2010;50:19–39. - PubMed
-
- Arostegui JI, Arnal C, Merino R, Modesto C, Antonia Carballo M, Moreno P, García-Consuegra J, Naranjo A, Ramos E, de Paz P, Rius J, Plaza S, Yagüe J. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum. 2007;56:3805–3813. - PubMed
-
- Banerjee D, Dick AD. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul. Immunol. Inflamm. 2004;12:115–25. - PubMed
-
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–6. - PubMed
-
- Broderick CA, Smith AJ, Balaggan KS, Georgiadis A, Buch PK, Trittibach PC, Barker SE, Sarra GM, Thrasher AJ, Dick AD, Ali RR. Local administration of an adeno-associated viral vector expressing IL-10 reduces monocyte infiltration and subsequent photoreceptor damage during experimental autoimmune uveitis. Mol. Ther. 2005;123:69–73. Erratum in:2006. 13, 829. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

