Smooth muscle myosin light chain kinase efficiently phosphorylates serine 15 of cardiac myosin regulatory light chain
- PMID: 22120626
- PMCID: PMC3242870
- DOI: 10.1016/j.bbrc.2011.11.044
Smooth muscle myosin light chain kinase efficiently phosphorylates serine 15 of cardiac myosin regulatory light chain
Abstract
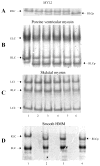
Specific phosphorylation of the human ventricular cardiac myosin regulatory light chain (MYL2) modifies the protein at S15. This modification affects MYL2 secondary structure and modulates the Ca(2+) sensitivity of contraction in cardiac tissue. Smooth muscle myosin light chain kinase (smMLCK) is a ubiquitous kinase prevalent in uterus and present in other contracting tissues including cardiac muscle. The recombinant 130 kDa (short) smMLCK phosphorylated S15 in MYL2 in vitro. Specific modification of S15 was verified using the direct detection of the phospho group on S15 with mass spectrometry. SmMLCK also specifically phosphorylated myosin regulatory light chain S15 in porcine ventricular myosin and chicken gizzard smooth muscle myosin (S20 in smooth muscle) but failed to phosphorylate the myosin regulatory light chain in rabbit skeletal myosin. Phosphorylation kinetics, measured using a novel fluorescence method eliminating the use of radioactive isotopes, indicates similar Michaelis-Menten V(max) and K(M) for regulatory light chain S15 phosphorylation rates in MYL2, porcine ventricular myosin, and chicken gizzard myosin. These data demonstrate that smMLCK is a specific and efficient kinase for the in vitro phosphorylation of MYL2, cardiac, and smooth muscle myosin. Whether smMLCK plays a role in cardiac muscle regulation or response to a disease causing stimulus is unclear but it should be considered a potentially significant kinase in cardiac tissue on the basis of its specificity, kinetics, and tissue expression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase.J Biol Chem. 2001 May 11;276(19):16365-73. doi: 10.1074/jbc.M011634200. Epub 2001 Feb 8. J Biol Chem. 2001. PMID: 11278951
-
Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo.J Biol Chem. 2010 Dec 24;285(52):40819-29. doi: 10.1074/jbc.M110.160499. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20943660 Free PMC article.
-
Substrate specificity of myosin light chain kinases.J Biol Chem. 1992 Dec 25;267(36):25945-50. J Biol Chem. 1992. PMID: 1464607
-
Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease.Gene. 2015 Sep 10;569(1):14-20. doi: 10.1016/j.gene.2015.06.027. Epub 2015 Jun 12. Gene. 2015. PMID: 26074085 Free PMC article. Review.
-
Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction.Circ J. 2009 Feb;73(2):208-13. doi: 10.1253/circj.cj-08-1041. Epub 2008 Dec 26. Circ J. 2009. PMID: 19110504 Review.
Cited by
-
Ventricular myosin modifies in vitro step-size when phosphorylated.J Mol Cell Cardiol. 2014 Jul;72:231-7. doi: 10.1016/j.yjmcc.2014.03.022. Epub 2014 Apr 12. J Mol Cell Cardiol. 2014. PMID: 24726887 Free PMC article.
-
Alcohol Use Disorders and Their Harmful Effects on the Contractility of Skeletal, Cardiac and Smooth Muscles.Adv Drug Alcohol Res. 2021 Oct;1:10011. doi: 10.3389/ADAR.2021.10011. Epub 2021 Oct 14. Adv Drug Alcohol Res. 2021. PMID: 35169771 Free PMC article.
-
Myosin regulatory light chain (RLC) phosphorylation change as a modulator of cardiac muscle contraction in disease.J Biol Chem. 2013 May 10;288(19):13446-54. doi: 10.1074/jbc.M113.455444. Epub 2013 Mar 25. J Biol Chem. 2013. PMID: 23530050 Free PMC article.
-
Analytical comparison of natural and pharmaceutical ventricular myosin activators.Biochemistry. 2014 Aug 19;53(32):5298-306. doi: 10.1021/bi500730t. Epub 2014 Aug 7. Biochemistry. 2014. PMID: 25068717 Free PMC article.
-
New Isoform of Cardiac Myosin Light Chain Kinase and the Role of Cardiac Myosin Phosphorylation in α1-Adrenoceptor Mediated Inotropic Response.PLoS One. 2015 Oct 29;10(10):e0141130. doi: 10.1371/journal.pone.0141130. eCollection 2015. PLoS One. 2015. PMID: 26512720 Free PMC article.
References
-
- Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287:H2712–H2718. - PubMed
-
- Szczesna-Cordary D. Regulatory Light Chains of Striated Muscle Myosin. Structure, Function and Malfunction Current Drug Targets-Cardiovascular & Haematological Disorders. 2003;3:187–197. - PubMed
-
- Stull JT, Nunnally MH, Moore RL, Blumenthal DK. Myosin light chain kinases and myosin phosphorylation in skeletal muscle. Adv Enzyme Regulation. 1985;23:123–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

