Cyclin E1 is a common target of BMI1 and MYCN and a prognostic marker for neuroblastoma progression
- PMID: 22120721
- PMCID: PMC3290747
- DOI: 10.1038/onc.2011.536
Cyclin E1 is a common target of BMI1 and MYCN and a prognostic marker for neuroblastoma progression
Abstract
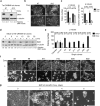
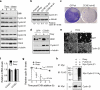
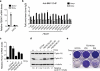
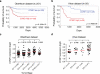
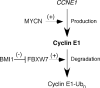
The Polycomb transcription repressor BMI1 is highly expressed in human neuroblastomas and is required for the clonogenic self-renewal and tumorigenicity of human neuroblastoma cell lines. The molecular basis of BMI1 action in neuroblastoma cells is not well understood. Here we report that BMI1 has a critical role in stabilizing cyclin E1 by repressing the expression of FBXW7, a substrate-recognition subunit of the SCF E3 ubiquitin ligase that targets cyclin E1 for degradation. BMI1 binds to the FBXW7 locus in vivo and represses its mRNA expression. Overexpression of cyclin E1 or abrogation of FBXW7 induction rescues the cell-death phenotype of BMI1 knockdown. Moreover, MYCN, an oncoprotein in the pathogenesis of high-risk neuroblastomas, is able to counteract the death-inducing effect of BMI1 knockdown by activating CCNE1 transcription. We further show that high cyclin E1 expression is associated with Stage 4 neuroblastomas and poor prognosis in patients. These findings suggest a molecular mechanism for the oncogenic activity of BMI1 and MYCN in neuroblastoma pathogenesis and progression by maintaining cyclin E1 levels.
Figures

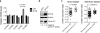
Similar articles
-
MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas.FASEB J. 2011 Dec;25(12):4138-49. doi: 10.1096/fj.11-185033. Epub 2011 Aug 19. FASEB J. 2011. PMID: 21856782 Free PMC article.
-
Bmi1 is a MYCN target gene that regulates tumorigenesis through repression of KIF1Bbeta and TSLC1 in neuroblastoma.Oncogene. 2010 May 6;29(18):2681-90. doi: 10.1038/onc.2010.22. Epub 2010 Mar 1. Oncogene. 2010. PMID: 20190806
-
Expression profiles and clinical relationships of ID2, CDKN1B, and CDKN2A in primary neuroblastoma.Genes Chromosomes Cancer. 2004 Dec;41(4):297-308. doi: 10.1002/gcc.20096. Genes Chromosomes Cancer. 2004. PMID: 15390183
-
Bmi1, stem cells, and senescence regulation.J Clin Invest. 2004 Jan;113(2):175-9. doi: 10.1172/JCI20800. J Clin Invest. 2004. PMID: 14722607 Free PMC article. Review.
-
The Fbxw7/hCdc4 tumor suppressor in human cancer.Cancer Lett. 2008 Nov 18;271(1):1-12. doi: 10.1016/j.canlet.2008.04.036. Epub 2008 Jun 9. Cancer Lett. 2008. PMID: 18541364 Review.
Cited by
-
Glycine decarboxylase is a transcriptional target of MYCN required for neuroblastoma cell proliferation and tumorigenicity.Oncogene. 2019 Dec;38(50):7504-7520. doi: 10.1038/s41388-019-0967-3. Epub 2019 Aug 23. Oncogene. 2019. PMID: 31444411 Free PMC article.
-
Rapamycin, a mTOR inhibitor, induced growth inhibition in retinoblastoma Y79 cell via down-regulation of Bmi-1.Int J Clin Exp Pathol. 2015 May 1;8(5):5182-8. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191215 Free PMC article.
-
Prognostic Values of CCNE1 Amplification and Overexpression in Cancer Patients: A Systematic Review and Meta-analysis.J Cancer. 2018 Jun 14;9(13):2397-2407. doi: 10.7150/jca.24179. eCollection 2018. J Cancer. 2018. PMID: 30026836 Free PMC article.
-
BMP4 and Neuregulin regulate the direction of mouse neural crest cell differentiation.Exp Ther Med. 2019 May;17(5):3883-3890. doi: 10.3892/etm.2019.7439. Epub 2019 Mar 26. Exp Ther Med. 2019. PMID: 31007733 Free PMC article.
-
The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma.Mol Brain. 2015 Oct 17;8(1):64. doi: 10.1186/s13041-015-0155-5. Mol Brain. 2015. PMID: 26475605 Free PMC article. Review.
References
-
- Asgharzadeh S, Pique-Regi R, Sposto R, Wang H, Yang Y, Shimada H, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–1203. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Chang H-H, Lee H, Hu M-K, Tsao P-N, Juan H-F, Huang M-C, et al. Notch1 Expression Predicts an Unfavorable Prognosis and Serves as a Therapeutic Target of Patients with Neuroblastoma. Clinical Cancer Research. 2010;16:4411–4420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

