Deprotonated imidodiphosphate in AMPPNP-containing protein structures
- PMID: 22120745
- PMCID: PMC3225179
- DOI: 10.1107/S0907444911046105
Deprotonated imidodiphosphate in AMPPNP-containing protein structures
Abstract
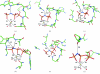
Many different proteins utilize the chemical energy provided by the cofactor adenosine triphosphate (ATP) for their proper function. A number of structures in the Protein Data Bank (PDB) contain adenosine 5'-(β,γ-imido)triphosphate (AMPPNP), a nonhydrolysable analog of ATP in which the bridging O atom between the two terminal phosphate groups is substituted by the imido function. Under mild conditions imides do not have acidic properties and thus the imide nitrogen should be protonated. However, an analysis of protein structures containing AMPPNP reveals that the imide group is deprotonated in certain complexes if the negative charges of the phosphate moieties in AMPPNP are in part neutralized by coordinating divalent metals or a guanidinium group of an arginine.
Figures
Similar articles
-
Structures of the Ca2+-ATPase complexes with ATP, AMPPCP and AMPPNP. An FTIR study.Biochim Biophys Acta. 2007 Jan;1767(1):114-23. doi: 10.1016/j.bbabio.2006.11.003. Epub 2006 Nov 11. Biochim Biophys Acta. 2007. PMID: 17157262
-
The conformations of a substrate and a product bound to the active site of S-adenosylmethionine synthetase.Biochemistry. 1999 Feb 23;38(8):2542-50. doi: 10.1021/bi9822933. Biochemistry. 1999. PMID: 10029549
-
X-ray structures of the MgADP, MgATPgammaS, and MgAMPPNP complexes of the Dictyostelium discoideum myosin motor domain.Biochemistry. 1997 Sep 30;36(39):11619-28. doi: 10.1021/bi9712596. Biochemistry. 1997. PMID: 9305951
-
A crystallographic glimpse of a nucleotide triphosphate (AMPPNP) bound to a protein surface: external and internal AMPPNP molecules in crystalline N-acetyl-L-glutamate kinase.Acta Crystallogr D Biol Crystallogr. 2002 Oct;58(Pt 10 Pt 2):1892-5. doi: 10.1107/s0907444902013689. Epub 2002 Sep 28. Acta Crystallogr D Biol Crystallogr. 2002. PMID: 12351849
-
Hydrolysis of AMPPNP by the motor domain of ncd, a kinesin-related protein.FEBS Lett. 1997 Jun 2;409(1):29-32. doi: 10.1016/s0014-5793(97)00472-9. FEBS Lett. 1997. PMID: 9199497
Cited by
-
AMPK targets a proto-oncogene TPD52 (isoform 3) expression and its interaction with LKB1 suppress AMPK-GSK3β signaling axis in prostate cancer.J Cell Commun Signal. 2023 Sep;17(3):957-974. doi: 10.1007/s12079-023-00745-y. Epub 2023 Apr 11. J Cell Commun Signal. 2023. PMID: 37040029 Free PMC article.
-
Preferential DNA Polymerase β Reverse Reaction with Imidodiphosphate.ACS Omega. 2020 Jun 19;5(25):15317-15324. doi: 10.1021/acsomega.0c01345. eCollection 2020 Jun 30. ACS Omega. 2020. PMID: 32637805 Free PMC article.
-
Extracellular ATP and Macropinocytosis: Their Interactive and Mutually Supportive Roles in Cell Growth, Drug Resistance, and EMT in Cancer.Subcell Biochem. 2022;98:61-83. doi: 10.1007/978-3-030-94004-1_4. Subcell Biochem. 2022. PMID: 35378703 Free PMC article. Review.
-
Getting the chemistry right: protonation, tautomers and the importance of H atoms in biological chemistry.Acta Crystallogr D Struct Biol. 2017 Feb 1;73(Pt 2):131-140. doi: 10.1107/S2059798316020283. Epub 2017 Feb 1. Acta Crystallogr D Struct Biol. 2017. PMID: 28177309 Free PMC article.
References
-
- Berry, M. B., Meador, B., Bilderback, T., Liang, P., Glaser, M. & Phillips, G. N. (1994). Proteins, 19, 183–198. - PubMed
-
- DeLano, W. L. (2002). PyMOL http://www.pymol.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

