Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury
- PMID: 22120983
- PMCID: PMC4986925
- DOI: 10.1002/hep.25500
Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury
Abstract
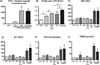
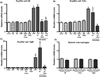
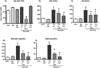
Infants with intestinal failure who are parenteral nutrition (PN)-dependent may develop cholestatic liver injury and cirrhosis (PN-associated liver injury: PNALI). The pathogenesis of PNALI remains incompletely understood. We hypothesized that intestinal injury with increased intestinal permeability combined with administration of PN promotes lipopolysaccharide (LPS)-Toll-like receptor 4 (TLR4) signaling dependent Kupffer cell (KC) activation as an early event in the pathogenesis of PNALI. We developed a mouse model in which intestinal injury and increased permeability were induced by oral treatment for 4 days with dextran sulphate sodium (DSS) followed by continuous infusion of soy lipid-based PN solution through a central venous catheter for 7 (PN7d/DSS) and 28 (PN28d/DSS) days. Purified KCs were probed for transcription of proinflammatory cytokines. PN7d/DSS mice showed increased intestinal permeability and elevated portal vein LPS levels, evidence of hepatocyte injury and cholestasis (serum aspartate aminotransferase, alanine aminotransferase, bile acids, total bilirubin), and increased KC expression of interleukin-6 (Il6), tumor necrosis factor α (Tnfα), and transforming growth factor β (Tgfβ). Markers of liver injury remained elevated in PN28d/DSS mice associated with lobular inflammation, hepatocyte apoptosis, peliosis, and KC hypertrophy and hyperplasia. PN infusion without DSS pretreatment or DSS pretreatment alone did not result in liver injury or KC activation, even though portal vein LPS levels were elevated. Suppression of the intestinal microbiota with broad spectrum antibiotics or ablation of TLR4 signaling in Tlr4 mutant mice resulted in significantly reduced KC activation and markedly attenuated liver injury in PN7d/DSS mice.
Conclusion: These data suggest that intestinal-derived LPS activates KC through TLR4 signaling in early stages of PNALI.
Copyright © 2012 American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA. 1968;203:860–864. - PubMed
-
- Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics. 1979;64:342–347. - PubMed
-
- Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–S28. - PubMed
-
- Kaufman SS, Loseke CA, Lupo JV, Young RJ, Murray ND, Pinch LW, et al. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr. 1997;131:356–361. - PubMed
-
- Pierro A, van Saene HK, Donnell SC, Hughes J, Ewan C, Nunn AJ, et al. Microbial translocation in neonates and infants receiving long-term parenteral nutrition. Arch Surg. 1996;131:176–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
