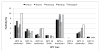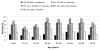Population-based human papillomavirus 16, 18, 6 and 11 DNA positivity and seropositivity in Chinese women
- PMID: 22120998
- PMCID: PMC4446973
- DOI: 10.1002/ijc.27367
Population-based human papillomavirus 16, 18, 6 and 11 DNA positivity and seropositivity in Chinese women
Abstract
To optimize HPV vaccination implementation at the population-level in China, data are needed on age-specific HPV 16, 18, 6 and 11 prevalence. This cross-sectional, population-based study evaluated the age- and type-specific HPV 16, 18, 6 and 11 prevalence of DNA and serum antibodies among women in China. From July 2006 to April 2007, 17-54 year old women from three rural provinces (Xinjiang, Shanxi and Henan) and two cities (Beijing and Shanghai) provided cervical exfoliated cells for HPV DNA and liquid-based cervical cytology (SurePath). High- and low-risk HPV types were detected with HC-II (Qiagen), with genotyping of HPV-positive samples using Linear Array (Roche). HPV 16, 18, 6 and 11 serum antibodies were detected using a Luminex-based, competitive immunoassay (Merck). A total of 4,206 women with DNA and serum antibody results were included. HPV 16 DNA prevalence peaked in women aged 30-34 (4.2%) and 45-49 yr (3.8%), while HPV 18 DNA prevalence peaked at ages 40-44 yr (1.3%). Most women were dually DNA and serum antibody negative: HPV 16 (92.2%), 18 (97.2%), HPV 16 and 18 (90.2%), 6 (92.0%), 11 (96.6%), 6 and 11(89.9%) and HPV 16, 18, 6 and 11 (82.5%). Future national HPV vaccination programs in China should target younger women due to increased exposure to HPV types 16, 18, 6 and 11 with increasing age. Cumulative exposure of HPV may be underreported in this population, as cross-sectional data do not accurately reflect exposure to HPV infections over time.
Copyright © 2011 UICC.
References
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type-distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Intl J Cancer. 2007;121:621–32. - PubMed
-
- Muñoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijder PJF, Meijer CJLM. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. - PubMed
-
- Garland SM, Steven M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural History of Genital Warts: Analysis of the Placebo Arm of 2 Randomized Phase III Trials of a Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Vaccine. J Infect Dis. 2009;199:805–14. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler Cm, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. - PubMed
-
- Muñoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Meyers E, Hood S, Bautista O, Bryan J, et al. Safety, immunogenicity and efficacy of quadrivalent human papillomavirus (types 6,11,16,18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous