Transition-metal-catalyzed denitrogenative transannulation: converting triazoles into other heterocyclic systems
- PMID: 22121072
- PMCID: PMC3393755
- DOI: 10.1002/anie.201104807
Transition-metal-catalyzed denitrogenative transannulation: converting triazoles into other heterocyclic systems
Abstract
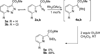
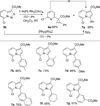
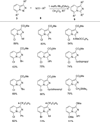
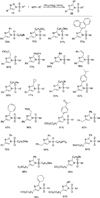
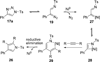
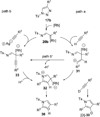
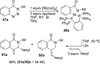
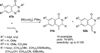
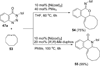
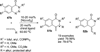
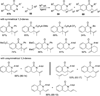
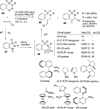
Transition metal catalyzed denitrogenative transannulation of a triazole ring has recently received considerable attention as a new concept for the construction of diverse nitrogen-containing heterocyclic cores. This method allows a single-step synthesis of complex nitrogen heterocycles from easily available and cheap triazole precursors. In this Minireview, recent progress of the transition metal catalyzed denitrogenative transannulation of a triazole ring, which was discovered in 2007, is discussed.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

