Transient expression of Ngn3 in Xenopus endoderm promotes early and ectopic development of pancreatic beta and delta cells
- PMID: 22121111
- PMCID: PMC3294191
- DOI: 10.1002/dvg.20828
Transient expression of Ngn3 in Xenopus endoderm promotes early and ectopic development of pancreatic beta and delta cells
Abstract
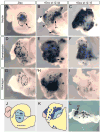
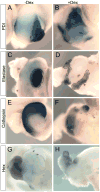
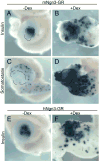
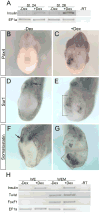
Promoting ectopic development of pancreatic beta cells from other cell types is one of the strategies being pursued for the treatment of diabetes. To achieve this, a detailed outline of the molecular lineage that operates in pancreatic progenitor cells to generate beta cells over other endocrine cell types is necessary. Here, we demonstrate that early transient expression of the endocrine progenitor bHLH protein Neurogenin 3 (Ngn3) favors the promotion of pancreatic beta and delta cell fates over an alpha cell fate, while later transient expression promotes ectopic development of all three endocrine cell fates. We found that short-term activation of Ngn3 in Xenopus laevis endoderm just after gastrulation was sufficient to promote both early and ectopic development of beta and delta cells. By examining gene expression changes 4 h after Ngn3 activation we identified several new downstream targets of Ngn3. We show that several of these are required for the promotion of ectopic beta cells by Ngn3 as well as for normal beta cell development. These results provide new detail regarding the Ngn3 transcriptional network operating in endocrine progenitor cells to specify a beta cell phenotype and should help define new approaches to promote ectopic development of beta cells for diabetes therapy.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


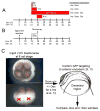
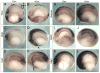
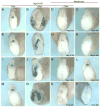
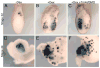
Similar articles
-
Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development.Development. 2010 Jan;137(2):203-12. doi: 10.1242/dev.041673. Development. 2010. PMID: 20040487 Free PMC article.
-
Pdx1 and Ngn3 overexpression enhances pancreatic differentiation of mouse ES cell-derived endoderm population.PLoS One. 2011;6(9):e24058. doi: 10.1371/journal.pone.0024058. Epub 2011 Sep 13. PLoS One. 2011. PMID: 21931641 Free PMC article.
-
neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1607-11. doi: 10.1073/pnas.97.4.1607. Proc Natl Acad Sci U S A. 2000. PMID: 10677506 Free PMC article.
-
Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration.Islets. 2009 Nov-Dec;1(3):177-84. doi: 10.4161/isl.1.3.9877. Islets. 2009. PMID: 21099270 Review.
-
Transcriptional regulation of α-cell differentiation.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:13-20. doi: 10.1111/j.1463-1326.2011.01440.x. Diabetes Obes Metab. 2011. PMID: 21824252 Review.
Cited by
-
Endogenous Pancreatic β Cell Regeneration: A Potential Strategy for the Recovery of β Cell Deficiency in Diabetes.Front Endocrinol (Lausanne). 2019 Feb 20;10:101. doi: 10.3389/fendo.2019.00101. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30842756 Free PMC article. Review.
-
Insm1 promotes endocrine cell differentiation by modulating the expression of a network of genes that includes Neurog3 and Ripply3.Development. 2014 Aug;141(15):2939-49. doi: 10.1242/dev.104810. Development. 2014. PMID: 25053427 Free PMC article.
-
Temporal induction of the homeodomain transcription factor Nkx2-1 is sufficient to respecify foregut and hindgut endoderm to a pulmonary fate in Xenopus laevis.MicroPubl Biol. 2025 May 7;2025:10.17912/micropub.biology.001610. doi: 10.17912/micropub.biology.001610. eCollection 2025. MicroPubl Biol. 2025. PMID: 40406581 Free PMC article.
-
Xenopus as a Model for GI/Pancreas Disease.Curr Pathobiol Rep. 2015 Jun 1;3(2):137-145. doi: 10.1007/s40139-015-0076-0. Curr Pathobiol Rep. 2015. PMID: 26236566 Free PMC article.
-
Transcriptional regulatory events initiated by Ascl1 and Neurog2 during neuronal differentiation of P19 embryonic carcinoma cells.J Mol Neurosci. 2015 Mar;55(3):684-705. doi: 10.1007/s12031-014-0408-2. Epub 2014 Sep 6. J Mol Neurosci. 2015. PMID: 25189318
References
-
- Amann JM, Chyla BJ, Ellis TC, Martinez A, Moore AC, Franklin JL, McGhee L, Meyers S, Ohm JE, Luce KS, Ouelette AJ, Washington MK, Thompson MA, King D, Gautam S, Coffey RJ, Whitehead RH, Hiebert SW. Mtgr1 is a transcriptional corepressor that is required for maintenance of the secretory cell lineage in the small intestine. Mol Cell Biol. 2005;25:9576–9585. - PMC - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe dA, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

