Concerted and adaptive alignment of decorin dermatan sulfate filaments in the graded organization of collagen fibrils in the equine superficial digital flexor tendon
- PMID: 22122012
- PMCID: PMC3275770
- DOI: 10.1111/j.1469-7580.2011.01456.x
Concerted and adaptive alignment of decorin dermatan sulfate filaments in the graded organization of collagen fibrils in the equine superficial digital flexor tendon
Abstract
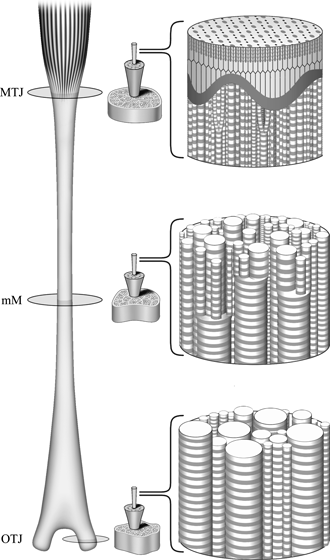
The equine superficial digital flexor tendon (SDFT) has a graded distribution of collagen fibril diameters, with predominantly small-diameter fibrils in the region of the myotendinous junction (MTJ), a gradual increase in large-diameter fibrils toward the osteotendinous junction (OTJ), and a mixture of small- and large-diameter fibrils in the middle metacarpal (MM) region. In this study, we investigated the ultrastructure of the SDFT, to correlate the spatial relationship of the collagen fibrils with the graded distribution. The surface-to-surface distances of pairs of fibrils were found to be almost constant over the entire tendon. However, the center-to-center distances varied according to fibril diameter. Decorin is the predominant proteoglycan in normal mature tendons, and has one dermatan sulfate (DS) or chondroitin sulfate (CS) filament as a side chain which is associated with the surfaces of the collagen fibrils via its core protein. We identified a coordinated arrangement of decorin DS filaments in the equine SDFT. The sizes of the decorin DS filaments detected by Cupromeronic blue staining showed a unique regional variation; they were shortest in the MM region and longer in the MTJ and OTJ regions, and a considerable number of filaments were arranged obliquely to adjacent collagen fibrils in the MTJ region. This regional variation of the filaments may be an adaptation to lubricate the interfibrillar space in response to local mechanical requirements. The results of this study suggest that the MTJ region, which receives the muscular contractile force first, acts as a buffer for mechanical forces in the equine SDFT.
© 2011 The Authors. Journal of Anatomy © 2011 Anatomical Society.
Figures


Similar articles
-
Graded arrangement of collagen fibrils in the equine superficial digital flexor tendon.Connect Tissue Res. 2007;48(6):332-7. doi: 10.1080/03008200701692800. Connect Tissue Res. 2007. PMID: 18075820
-
Control of the collagen fibril diameter in the equine superficial digital flexor tendon in horses by decorin.J Vet Med Sci. 2005 Sep;67(9):855-60. doi: 10.1292/jvms.67.855. J Vet Med Sci. 2005. PMID: 16210795
-
Characteristics of collagen fibrils in the entire equine superficial digital flexor tendon.Okajimas Folia Anat Jpn. 2007 Nov;84(3):111-4. doi: 10.2535/ofaj.84.111. Okajimas Folia Anat Jpn. 2007. PMID: 18186224
-
Collagen structure of tendon relates to function.ScientificWorldJournal. 2007 Mar 30;7:404-20. doi: 10.1100/tsw.2007.92. ScientificWorldJournal. 2007. PMID: 17450305 Free PMC article. Review.
-
Proteoglycan: collagen interactions in connective tissues. Ultrastructural, biochemical, functional and evolutionary aspects.Int J Biol Macromol. 1991 Jun;13(3):157-61. doi: 10.1016/0141-8130(91)90041-r. Int J Biol Macromol. 1991. PMID: 1911556 Review.
Cited by
-
Focal experimental injury leads to widespread gene expression and histologic changes in equine flexor tendons.PLoS One. 2015 Apr 2;10(4):e0122220. doi: 10.1371/journal.pone.0122220. eCollection 2015. PLoS One. 2015. PMID: 25837713 Free PMC article.
-
Sequence analysis and domain motifs in the porcine skin decorin glycosaminoglycan chain.J Biol Chem. 2013 Mar 29;288(13):9226-37. doi: 10.1074/jbc.M112.437236. Epub 2013 Feb 19. J Biol Chem. 2013. PMID: 23423381 Free PMC article.
-
Ring-Mesh Model of Proteoglycan Glycosaminoglycan Chains in Tendon based on Three-dimensional Reconstruction by Focused Ion Beam Scanning Electron Microscopy.J Biol Chem. 2016 Nov 4;291(45):23704-23708. doi: 10.1074/jbc.M116.733857. Epub 2016 Sep 13. J Biol Chem. 2016. PMID: 27624935 Free PMC article.
-
Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon.J Biomech Eng. 2013 Feb;135(2):021019. doi: 10.1115/1.4023234. J Biomech Eng. 2013. PMID: 23445064 Free PMC article.
-
Collagen V expression is crucial in regional development of the supraspinatus tendon.J Orthop Res. 2016 Dec;34(12):2154-2161. doi: 10.1002/jor.23246. Epub 2016 Apr 7. J Orthop Res. 2016. PMID: 28005290 Free PMC article.
References
-
- Adachi E, Hayashi T. Anchoring of epithelia to underlying connective tissue: evidence of frayed ends of collagen fibrils directly merging with meshwork of lamina densa. J Electron Microsc (Tokyo) 1994;43:264–271. - PubMed
-
- Basalo IM, Chahine NO, Kaplun M, et al. Chondroitin sulfate reduces the friction coefficient of articular cartilage. J Biomech. 2007;40:1847–1854. - PubMed
-
- Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular composition. Equine Vet J. 1998;30:534–539. - PubMed
-
- Elliott DM, Robinson PS, Gimbel JA, et al. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31:599–605. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

