γ-Secretase inhibitors and modulators for Alzheimer's disease
- PMID: 22122056
- PMCID: PMC3254709
- DOI: 10.1111/j.1471-4159.2011.07501.x
γ-Secretase inhibitors and modulators for Alzheimer's disease
Abstract
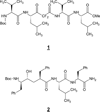
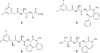
γ-Secretase is a membrane embedded aspartyl protease complex with presenilin as the catalytic component. Along with β-secretase, this enzyme produces the amyloid β-protein of Alzheimer's disease (AD) from the amyloid β-protein precursor. Because of its key role in the pathogenesis of AD, γ-secretase has been a prime target for drug discovery, and many inhibitors of this protease have been developed. The therapeutic potential of these inhibitors is virtually negated by the fact that γ-secretase is an essential part of the Notch signaling pathway, rendering the compounds unacceptably toxic upon chronic exposure. However, these compounds have served as useful chemical tools for biological investigations. In contrast, γ-secretase modulators continue to be of keen interest as possible AD therapeutics. These modulators either shift amyloid β-protein production to shorter, less pathogenic peptides or inhibit the proteolysis of amyloid β-protein precursor selectively compared to that of Notch. The various chemical types of inhibitors and modulators will be discussed, along with their use as probes for basic biology and their potential as AD therapeutics.
© 2011 The Author. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Conflict of interest statement
The author declares no conflict of interests.
Figures
References
-
- Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem. 2004;279:43419–43426. - PubMed
-
- Beher D, Fricker M, Nadin A, et al. In vitro characterization of the presenilin-dependent gamma-secretase complex using a novel affinity ligand. Biochemistry. 2003;42:8133–8142. - PubMed
-
- Bihel F, Das C, Bowman MJ, Wolfe MS. Discovery of a subnanomolar helical D-tridecapeptide inhibitor of γ-secretase. J Med Chem. 2004;47:3931–3933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical