Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum
- PMID: 22122410
- PMCID: PMC3237906
- DOI: 10.1111/j.1460-9568.2011.07914.x
Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum
Abstract
Phasic changes in dopamine activity play a critical role in learning and goal-directed behavior. Unpredicted reward and reward-predictive cues evoke phasic increases in the firing rate of the majority of midbrain dopamine neurons--results that predict uniformly broadcast increases in dopamine concentration throughout the striatum. However, measurement of dopamine concentration changes during reward has cast doubt on this prediction. We systematically measured phasic changes in dopamine in four striatal subregions [nucleus accumbens shell and core (Core), dorsomedial (DMS) and dorsolateral striatum] in response to stimuli known to activate a majority of dopamine neurons. We used fast-scan cyclic voltammetry in awake and behaving rats, which measures changes in dopamine on a similar timescale to the electrophysiological recordings that established a relationship between phasic dopamine activity and reward. Unlike the responses of midbrain dopamine neurons, unpredicted food reward and reward-predictive cues evoked a phasic increase in dopamine that was subregion specific. In rats with limited experience, unpredicted food reward evoked an increase exclusively in the Core. In rats trained on a discriminative stimulus paradigm, both unpredicted reward and reward-predictive cues evoked robust phasic dopamine in the Core and DMS. Thus, phasic dopamine release in select target structures is dynamic and dependent on context and experience. Because the four subregions assayed receive different inputs and have differential projection targets, the regional selectivity of phasic changes in dopamine has important implications for information flow through the striatum and plasticity that underlies learning and goal-directed behavior.
© 2011 The Authors. European Journal of Neuroscience © 2011 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
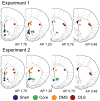



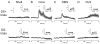

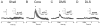
References
-
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

