Redox-assisted protein folding systems in eukaryotic parasites
- PMID: 22122448
- PMCID: PMC3373220
- DOI: 10.1089/ars.2011.4433
Redox-assisted protein folding systems in eukaryotic parasites
Abstract
Significance: The cysteine (Cys) residues of proteins play two fundamentally important roles. They serve as sites of post-translational redox modifications as well as influence the conformation of the protein through the formation of disulfide bonds.
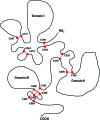
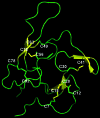
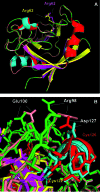
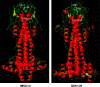
Recent advances: Redox-related and redox-associated protein folding in protozoan parasites has been found to be a major mode of regulation, affecting myriad aspects of the parasitic life cycle, host-parasite interactions, and the disease pathology. Available genome sequences of various parasites have begun to complement the classical biochemical and enzymological studies of these processes. In this article, we summarize the reversible Cys disulfide (S-S) bond formation in various classes of strategically important parasitic proteins, and its structural consequence and functional relevance.
Critical issues: Molecular mechanisms of folding remain under-studied and often disconnected from functional relevance.
Future directions: The clinical benefit of redox research will require a comprehensive characterization of the various isoforms and paralogs of the redox enzymes and their concerted effect on the structure and function of the specific parasitic client proteins.
Figures




References
-
- Adisa A. Rug M. Foley M. Tilley L. Characterisation of a delta-COP homologue in the malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 2002;123:11–21. - PubMed
-
- Agorio A. Chalar C. Cardozo S. Salinas G. Alternative mRNAs arising from trans- splicing code for mitochondrial and cytosolic variants of Echinococcus granulosus thioredoxin glutathione reductase. J Biol Chem. 2003;278:12920–12928. - PubMed
-
- Aguilar-Díaz H. Carrero JC. Argüello-García R. Laclette JP. Morales-Montor J. Cyst and encystment in protozoan parasites: optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011;27:450–458. - PubMed
-
- Alger HM. Williams DL. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol Biochem Parasitol. 2002;121:129–139. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

