Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands
- PMID: 22122563
- PMCID: PMC3381613
- DOI: 10.1021/jm201436k
Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands
Abstract
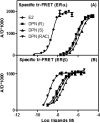
Two estrogen receptor (ER) subtypes, ERα and ERβ, mediate the actions of estrogens in diverse reproductive and nonreproductive target tissues. ER subtype-selective ligands, which bind to and activate these subtypes differentially, have proved to be useful in elucidating which actions of estrogens proceed through ERα vs ERβ. Some of these ligands show potential as novel therapeutic agents. Diarylpropionitrile (DPN), an ERβ selective ligand that we developed, is a chiral molecule, but it has been studied almost exclusively as the racemic mixture (rac-DPN, 1). Herein we report the development of an efficient enantioselective synthesis of the two isomers, R-DPN (3) and S-DPN (2), and we have compared the in vitro ligand binding affinities, coactivator binding affinities, recruitment potencies, and cellular transcriptional potencies of these isomers. Both enantiomers show a very high affinity and potency preference for ERβ over ERα, typically in the range of 80-300-fold. Although the enantioselectivity is only modest (3-4-fold), the R-enantiomer is the higher affinity and more potent isomer. While ERβ can be effectively and selectively stimulated by rac-DPN or by either R-DPN or S-DPN, R-DPN might be the preferred member of this isomeric series for biological studies of ERβ function.
Figures



References
-
- McDonnell DP. The Molecular Pharmacology of SERMs. Trends Endocrinol Metab. 1999;10:301–311. - PubMed
-
- McDonnell DP. Selective estrogen receptor modulators (SERMs): A first step in the development of perfect hormone replacement therapy regimen. J Soc Gynecol Investig. 2000;7:S10–S15. - PubMed
-
- Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. - PubMed
-
- Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

