The use of carer assisted adherence therapy for people with Parkinson's disease and their carers (CAAT-PARK): study protocol for a randomised controlled trial
- PMID: 22122912
- PMCID: PMC3235063
- DOI: 10.1186/1745-6215-12-251
The use of carer assisted adherence therapy for people with Parkinson's disease and their carers (CAAT-PARK): study protocol for a randomised controlled trial
Abstract
Background: Pharmacological intervention is essential for managing the symptoms of Parkinson's disease. Adherence to medication regimens however is a major problem. Poor adherence leads to significant motor deterioration and inadequate symptom control. This results in poor quality of life. Whilst interventions to improve medication adherence have shown considerable benefit in other chronic conditions, the efficacy of such treatments in Parkinson's disease is less well researched. Many people with Parkinson's disease require substantial support from spouse/caregivers. This often extends to medication taking. Consequently, spouse/caregiver's support for timely medication management is paramount. We aim to investigate the benefit of a novel intervention, Carer Assisted Adherence Therapy, for improving medication adherence and quality of life in people with Parkinson's disease. Adherence therapy may help to optimise the efficacy of anti-parkinsonian agents, subsequently improving clinical outcomes.
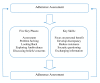
Methods/design: A parallel, randomised controlled trial will be conducted to investigate whether carer assisted adherence therapy is effective for improving medication adherence and quality of life. We aim to recruit 40 patient/carer pairs into each group. Participants will be randomly assigned by the Clinical Research Trials Unit at the University of East Anglia. Adherence therapy is a brief cognitive-behavioural approach aimed at facilitating a process of shared decision making. The central theory is that when patients make shared choices with a professional they are more likely to continue with those choices because they are personally owned and meaningful. Outcomes will be rates of adherence and quality of life, determined by the Morisky Medication Adherence Scale-4 and the Parkinson's disease Questionnaire-39 respectively. Assessments will take place post randomisation, immediately post intervention and 12-weeks post randomisation. Primary outcomes are adherence and quality of life at 12-week follow-up. Efficacy will be determined using intention-to-treat analysis. Independent samples t-tests will compare mean changes between groups from baseline to follow-up. Per protocol analysis will be conducted based on individuals with no major protocol deviation. Where imbalances in baseline characteristics are identified, an adjusted analysis will be performed using a regression model. Analysis will be masked to treatment allocation.
Trial registration: ISRCTN: ISRCTN07830951.
Figures
Similar articles
-
Smartphone- and internet-assisted self-management and adherence tools to manage Parkinson's disease (SMART-PD): study protocol for a randomised controlled trial (v7; 15 August 2014).Trials. 2014 Sep 25;15:374. doi: 10.1186/1745-6215-15-374. Trials. 2014. PMID: 25257518 Free PMC article. Clinical Trial.
-
Adherence therapy improves medication adherence and quality of life in people with Parkinson's disease: a randomised controlled trial.Int J Clin Pract. 2014 Aug;68(8):963-71. doi: 10.1111/ijcp.12439. Epub 2014 Apr 20. Int J Clin Pract. 2014. PMID: 24750544 Clinical Trial.
-
Cognitive rehabilitation for Parkinson’s disease dementia: a study protocol for a pilot randomised controlled trial.Trials. 2016 Mar 22;17:152. doi: 10.1186/s13063-016-1253-0. Trials. 2016. PMID: 27000036 Free PMC article. Clinical Trial.
-
Medication Adherence in People With Parkinson Disease.J Neurosci Nurs. 2016 Jul-Aug;48(4):185-94. doi: 10.1097/JNN.0000000000000198. J Neurosci Nurs. 2016. PMID: 27224682 Review.
-
Medication adherence in patients with Parkinson's disease.CNS Drugs. 2015 Jan;29(1):47-53. doi: 10.1007/s40263-014-0220-0. CNS Drugs. 2015. PMID: 25503824 Review.
Cited by
-
Medication nonadherence in Parkinson's disease.Curr Neurol Neurosci Rep. 2013 Oct;13(10):382. doi: 10.1007/s11910-013-0382-z. Curr Neurol Neurosci Rep. 2013. PMID: 23954970 Free PMC article. Review. No abstract available.
-
Qualitative evaluation of adherence therapy in Parkinson's disease: a multidirectional model.Patient Prefer Adherence. 2015 Jul 10;9:989-98. doi: 10.2147/PPA.S80158. eCollection 2015. Patient Prefer Adherence. 2015. PMID: 26203231 Free PMC article.
-
Medication Therapy Management Service for Patients with Parkinson's Disease: A Before-and-After Study.Neurol Ther. 2016 Jun;5(1):85-99. doi: 10.1007/s40120-016-0046-4. Epub 2016 Jun 7. Neurol Ther. 2016. PMID: 27271736 Free PMC article.
-
Smartphone- and internet-assisted self-management and adherence tools to manage Parkinson's disease (SMART-PD): study protocol for a randomised controlled trial (v7; 15 August 2014).Trials. 2014 Sep 25;15:374. doi: 10.1186/1745-6215-15-374. Trials. 2014. PMID: 25257518 Free PMC article. Clinical Trial.
References
-
- NICE. The National Collaborating Centre for Chronic Conditions. Parkinson's Disease: National clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians; 2006. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical