Protein separation by capillary gel electrophoresis: a review
- PMID: 22122927
- PMCID: PMC3227876
- DOI: 10.1016/j.aca.2011.10.022
Protein separation by capillary gel electrophoresis: a review
Abstract
Capillary gel electrophoresis (CGE) has been used for protein separation for more than two decades. Due to the technology advancement, current CGE methods are becoming more and more robust and reliable for protein analysis, and some of the methods have been routinely used for the analysis of protein-based pharmaceuticals and quality controls. In light of this progress, we survey 147 papers related to CGE separations of proteins and present an overview of this technology. We first introduce briefly the early development of CGE. We then review the methodology, in which we specifically describe the matrices, coatings, and detection strategies used in CGE. CGE using microfabricated channels and incorporation of CGE with two-dimensional protein separations are also discussed in this section. We finally present a few representative applications of CGE for separating proteins in real-world samples.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
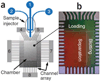

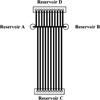
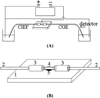

References
-
- Shapiro AL, Vinuela E, Maizel JV. Biochem. Biophys. Res. Commun. 1967;28:815–820. - PubMed
-
- Weber K, Osborn M. J. Biol. Chem. 1969;244:4406–4412. - PubMed
-
- Guttman A, Nolan J. Anal. Biochem. 1994;221:285–289. - PubMed
-
- Shieh PCH, Hoang D, Guttman A, Cooke N, J Chromatogr A. 1994;676:219–226.
-
- Guttman A. TrAC Trends Anal. Chem. 1996;15:194–198.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

