Chemical characterization of some substituted hydroxyapatites
- PMID: 22122971
- PMCID: PMC3284456
- DOI: 10.1186/1752-153X-5-74
Chemical characterization of some substituted hydroxyapatites
Abstract
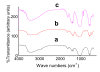
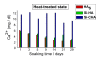
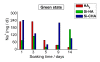
Synthetic multi-substituted hydroxyapatite nano powders containing silicon and or carbonate prepared by a wet chemical method. The process parameters are set up to allow the simultaneous substitution of carbonate and silicon ions in the place of phosphorus. The chemical and structural characterizations of the prepared powders are determined with the aid of; XRF, ICP, XRD and FTIR. The results show that, the ion substitution in the crystal lattice of HA caused a change in the unit cell dimensions and affected the degree of crystallization of the produced powders. The apatite formation abilityy of the prepared discs from the synthesized powders is determined by immersing in SBF solution for different periods. The degree of ion release was determined in the obtained solutions. The examined surface of the immersed discs under SEM and analyzed by CDS showed a more dense HA layer than those of un-substituted ones. The HA with the substituted silicon and carbonate ions, showed the highest solubility with greater rate of ion release, compared with carbonate-free powder. All prepared powders took sodium ion from the SBF solution during immersion, which was not recorded before.
Figures















References
-
- Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mate Res. 1998;13:94–117. doi: 10.1557/JMR.1998.0015. - DOI
-
- Driessens FCM. The mineral in bone, dentin and tooth enamel. Bull Soc Chim Belg. 1997;89:663–689.
-
- Smith DK. In: Hydroxyapatite and Related Materials. Brown PW, Constantz B, editor. Boca Raton, FL: CRC Press; 1994. Calcium Phosphate Apatites in Nature; pp. 29–45.
-
- Carlisle EM. Silicon: a possible factor in bone calcification. Science. 1969;167:279–80. - PubMed
LinkOut - more resources
Full Text Sources

