Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility
- PMID: 22122978
- PMCID: PMC3377919
- DOI: 10.1186/1754-6834-4-54
Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility
Abstract
Background: In recent years, biorefining of lignocellulosic biomass to produce multi-products such as ethanol and other biomaterials has become a dynamic research area. Pretreatment technologies that fractionate sugarcane bagasse are essential for the successful use of this feedstock in ethanol production. In this paper, we investigate modifications in the morphology and chemical composition of sugarcane bagasse submitted to a two-step treatment, using diluted acid followed by a delignification process with increasing sodium hydroxide concentrations. Detailed chemical and morphological characterization of the samples after each pretreatment condition, studied by high performance liquid chromatography, solid-state nuclear magnetic resonance, diffuse reflectance Fourier transformed infrared spectroscopy and scanning electron microscopy, is reported, together with sample crystallinity and enzymatic digestibility.
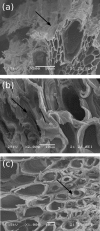
Results: Chemical composition analysis performed on samples obtained after different pretreatment conditions showed that up to 96% and 85% of hemicellulose and lignin fractions, respectively, were removed by this two-step method when sodium hydroxide concentrations of 1% (m/v) or higher were used. The efficient lignin removal resulted in an enhanced hydrolysis yield reaching values around 100%. Considering the cellulose loss due to the pretreatment (maximum of 30%, depending on the process), the total cellulose conversion increases significantly from 22.0% (value for the untreated bagasse) to 72.4%. The delignification process, with consequent increase in the cellulose to lignin ratio, is also clearly observed by nuclear magnetic resonance and diffuse reflectance Fourier transformed infrared spectroscopy experiments. We also demonstrated that the morphological changes contributing to this remarkable improvement occur as a consequence of lignin removal from the sample. Bagasse unstructuring is favored by the loss of cohesion between neighboring cell walls, as well as by changes in the inner cell wall structure, such as damaging, hole formation and loss of mechanical resistance, facilitating liquid and enzyme access to crystalline cellulose.
Conclusions: The results presented herewith show the efficiency of the proposed method for improving the enzymatic digestibility of sugarcane bagasse and provide understanding of the pretreatment action mechanism. Combining the different techniques applied in this work warranted thorough information about the undergoing morphological and chemical changes and was an efficient approach to understand the morphological effects resulting from sample delignification and its influence on the enhanced hydrolysis results.
Figures










Similar articles
-
Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse.Biotechnol Biofuels. 2015 Dec 2;8:205. doi: 10.1186/s13068-015-0384-y. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 26633992 Free PMC article.
-
Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: a potentially valuable source of fermentable sugars for biofuel production - part 1.Biotechnol Biofuels. 2013 May 9;6(1):75. doi: 10.1186/1754-6834-6-75. Biotechnol Biofuels. 2013. PMID: 23657132 Free PMC article.
-
Nuclear magnetic resonance investigation of water accessibility in cellulose of pretreated sugarcane bagasse.Biotechnol Biofuels. 2014 Sep 10;7(1):127. doi: 10.1186/s13068-014-0127-5. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25342969 Free PMC article.
-
Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective.Int J Biol Macromol. 2021 Feb 1;169:564-582. doi: 10.1016/j.ijbiomac.2020.12.175. Epub 2020 Dec 29. Int J Biol Macromol. 2021. PMID: 33385447 Review.
-
Oxidative pretreatment of lignocellulosic biomass for enzymatic hydrolysis: Progress and challenges.Bioresour Technol. 2023 Jan;367:128208. doi: 10.1016/j.biortech.2022.128208. Epub 2022 Oct 30. Bioresour Technol. 2023. PMID: 36323374 Review.
Cited by
-
Analysis of the Transcriptome in Aspergillus tamarii During Enzymatic Degradation of Sugarcane Bagasse.Front Bioeng Biotechnol. 2018 Sep 18;6:123. doi: 10.3389/fbioe.2018.00123. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30280097 Free PMC article.
-
Improving the enzymatic hydrolysis of thermo-mechanical fiber from Eucalyptus urophylla by a combination of hydrothermal pretreatment and alkali fractionation.Biotechnol Biofuels. 2014 Aug 20;7(1):116. doi: 10.1186/s13068-014-0116-8. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25184000 Free PMC article.
-
Systematic studies of the interactions between a model polyphenol compound and microbial β-glucosidases.PLoS One. 2017 Jul 20;12(7):e0181629. doi: 10.1371/journal.pone.0181629. eCollection 2017. PLoS One. 2017. PMID: 28727856 Free PMC article.
-
Conformational changes in a hyperthermostable glycoside hydrolase: enzymatic activity is a consequence of the loop dynamics and protonation balance.PLoS One. 2015 Feb 27;10(2):e0118225. doi: 10.1371/journal.pone.0118225. eCollection 2015. PLoS One. 2015. PMID: 25723179 Free PMC article.
-
A Substantial Role of Agro-Textiles in Agricultural Applications.Front Plant Sci. 2022 Jun 21;13:895740. doi: 10.3389/fpls.2022.895740. eCollection 2022. Front Plant Sci. 2022. PMID: 35800605 Free PMC article. Review.
References
-
- Report on sugarcane production from Sugarcane Industry Association (UNICA - Brazil) http://english.unica.com.br/dadosCotacao/estatistica/
-
- Betancur GJV, Pereira N Jr. Sugar cane bagasse as feedstock for second generation ethanol production. Part I: diluted acid pretreatment optimization. Electron J Biotechnol. 2010;13:1–9.
LinkOut - more resources
Full Text Sources

